
In the previous article, we shared the diseases related to ADAMTS5 gene mutations, such as osteoarthritis (OA) . Human osteoarthritis is a progressive disease of the joints characterized by degradation of articular cartilage. Researchers perform mechanism of action (MOA) studies to link genes to disease phenotypes. Exemplified by the study of ADAMTS5 described herein, the application of mouse models accelerates the understanding of disease mechanisms for translational research.
To explore which proteoglycans are responsible for the degradation of cartilage during the onset of human osteoarthritis (OA), Sonya S. Glasson and team addressed this question using genetically modified mouse models. Specifically, they implemented gene-targeted deletion of the catalytic domains of ADAMTS4 or ADAMTS5 in mice, followed by induction of cartilage degradation by surgically induced joint instability. The functions of ADAMTS4 and 5 activity in normal development and physiology were also investigated by analysis of the histological appearance of organs in adult animals.
The ADAMTS5 (ADAMTS5 -/-) knockout was created by cre/lox mediated recombination, resulting in the deletion of 56 amino acids encoded by exon 3, including disruption of the zinc-binding site and deletion of the conserved ‘Met-turn’ (Fig. 1a). The deletion was confirmed by polymerase chain reaction (PCR) from tail DNA (Fig. 1b). PCR with reverse transcription (RT–PCR) from spleen total RNA gave PCR products consistent with an ADAMTS5 messenger RNA lacking exon 3 (Fig. 1c). The presence of ADAMTS5 mRNA was confirmed in the articular cartilage (Fig. 1d), but the presence of the translated protein lacking the catalytic domain could not be confirmed in either wild-type or ADAMTS5 -/- articular cartilage.
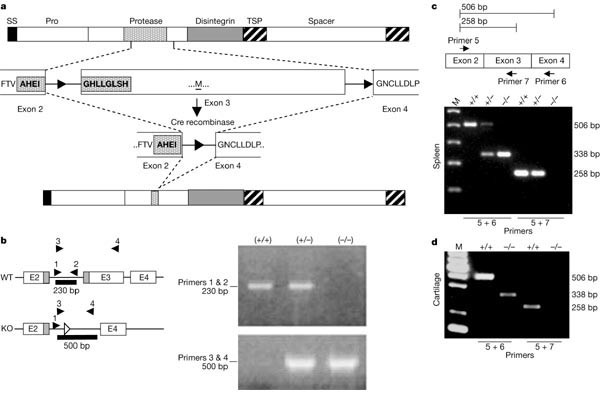
Figure 1. Targeted disruption of the murine ADAMTS5 gene. [2]
They used two scoring reporting systems to score knockout and wild-type mice. Scores were reported as mean maximal scores from wild-type, ADAMTS5+/- and ADAMTS5-/- mice. In addition, the scores from each section through the joint were added together, to express the severity of pathology as the summed scores for each treatment group at both 4 and 8 weeks after surgery. This second method of scoring takes into account severity of the lesion as well as the surface area of the joint affected. Both methods of analysis revealed a significant reduction (P < 0.05) in the scores of the ADAMTS5+/- and ADAMTS5-/- mice compared with wild-type mice (Fig. 2). The summed scores of the ADAMTS5 knockout animals were less than 50% of the wild type, mainly as a result of reduced severity and surface area involvement. The severity of disease in heterozygous knockout animals was also significantly reduced 8 weeks after induction of joint instability.

Figure 2. Histological scores of joints 4 and 8 weeks after induction of joint instability. [2]
Mice with a targeted deletion of the catalytic domain of ADAMTS-5 were protected from cartilage loss in the destabilization of the medial meniscus (DMM) surgical models, with minimal cleavage of aggrecan at the Glu392↓Ala393 bond. Moreover, a significant cleavage in the CS-2 domain was observed in these mice, suggesting an involvement of ADAMTS-5 in pathological aggrecan proteolysis rather than physiological turnover. [2]
A lot of research still needs to be done to uncover the multiple biological processes orchestrated by metalloproteinases. The history of ADAMTS-5 research shows this in a dramatic way. In a few years, ADAMTS-5 evolved from an OA target to also serve as a cardiovascular target candidate, while additional unpredicted biological roles were uncovered by analysing the phenotypes of Adamts5 knockout mice.
Pharmaceutical companies are still actively pursuing ADAMTS-5 as a therapeutic target for OA. Some drugs have shown side effects in clinical trials or are inconclusive, but many relevant researchers still have confidence in this approach. After all, it is quite possible that those effects were drug-related rather than target-related.
At present, the adverse side effects are observed in Cynomolgus upon administration of GSK2394002.[3] Agg-523, Wyeth's ADAMTS-4/ADAMTS-5 small molecule inhibitor, was investigated in phase I clinical trials in patients with mild to moderate and severe knee OA (NCT00454298 and NCT00427687), but no results are currently available. Another orally available small molecule inhibitor of ADAMTS-5 developed by Galapagos NV, GLPG1972, with a modest (5-fold) selectivity over ADAMTS-4, is protective against surgically induced OA in mice and rats. [4]
Whatever the outcome of these clinical trials, there are a few problems that ADAMTS-5 targeting therapies should address in a clinically relevant context. For example, administration of ADAMTS-5 inhibitors may require a careful follow-up in OA patients, as they may make them more susceptible to influenza infection and adverse cardiovascular effects, such as aortic aneurysms, myxomatous valves, and atherosclerosis. Given the administration of ADAMTS-5 inhibitors to an aged patient population with multiple comorbidities, it is important to fully understand their effects beyond cartilage degradation alone. Moreover, an important application of ADAMTS-5 therapy may be on young athletes with post-traumatic OA, who may better respond to pharmacological treatment.
Reference:
[1] Glasson S S, Askew R, Sheppard B, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis[J]. Nature, 2005, 434(7033): 644-648.
[2] Santamaria S. ADAMTS‐5: A difficult teenager turning 20[J]. International journal of experimental pathology, 2020, 101(1-2): 4-20.
[3] Larkin J, Lohr T, Elefante L, et al. The highs and lows of translational drug development: antibody-mediated inhibition of ADAMTS-5 for osteoarthritis disease modification[J]. Osteoarthritis and Cartilage, 2014, 22: S483-S484.
[4] Clement-Lacroix P, Little C, Meurisse S, et al. GLPG1972: a potent, selective, orally available adamts-5 inhibitor for the treatment of OA[J]. Osteoarthritis and Cartilage, 2017, 25: S58-S59.
We will respond to you in 1-2 business days.