

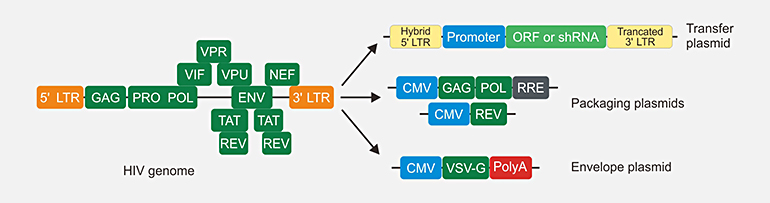
Self-inactivating VSV-G pseudotyped lentivirus
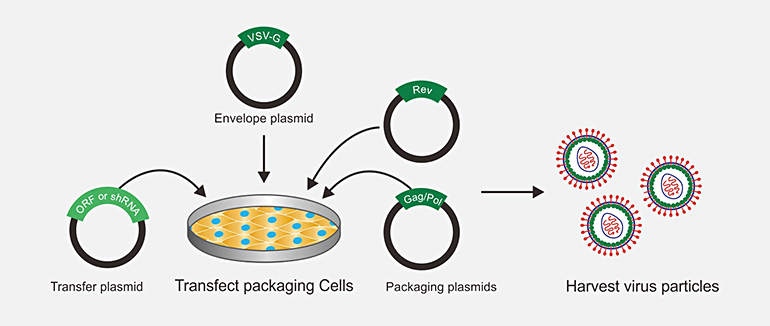
Transfect 293T packaging cells with transfer plasmid, envelope plasmid and packaging plasmids. Remove media, replace with fresh media. After 48~72 hours, harvest virus. Harvested virus can be used in vitro study. After further purification, ultra-purified lentivirus can be used in vivo study.
Lentiviral titer test will be conducted to make sure only qualified lentivirus entering into your laboratory. For luciferase reporter gene labeled lentivirus, we will perform viral transduction assay and further confirm the titer by qPCR.
| Lentivirus Packaging | Deliverable | Turnaraound | Application |
| Standard-scale | Lentivirus (Titer: ≥1×108 TU/ml, Volume: 1ml) |
2-3 weeks | Cell transduction |
| Large-scale | Lentivirus (Titer: ≥1×109 TU/ml, Volume: 1ml) |
2-3 weeks | Cell transduction |
Cyagen can also provide custom shRNA and gRNA lentivirus packaging services.
The Targeted Gene Editing system can be used for knocking out gene expression in vivo or in vitro. By using lentiviral-based Targeted Gene Editing constructs, expressed gRNA and Targeted Gene Editing can be used in dividing or non-dividing cells or whole model organisms.
Two vector Targeted Gene Editing system: Lentiviral gRNA vector and Lentiviral Targeted Gene Editing vector.
1. Lentiviral-based gRNA Vector
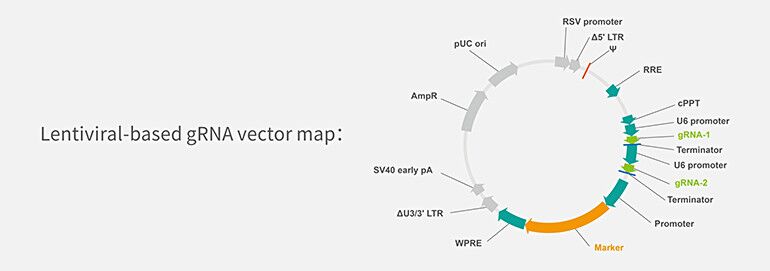
Lentiviral gRNA vector consists of lentiviral genome sequence from 5’ LTR to 3’ LTR and bacterial plasmid sequence which contains an Ampicillin selection cassette and a pUC origin of replication.
2. Lentiviral-based Targeted Gene Editing Vector
CBh promoter drives co-expression of spTargeted Gene Editing and resistance gene.
For two vector Targeted Gene Editing system, two different drug selection cassettes will be used to select gRNA and Targeted Gene Editing co-expressed clones.
We will perform sequencing assay as well as restriction enzyme analysis for every project to make sure only qualified vectors enter your laboratory.
Request a quote now. Alternatively, you can always email service-apac@cyagen.com to inquire about our services or obtain a quote for your project.