
Immune checkpoint proteins (ICP) are a group of signaling molecules possessed by immune cells that can regulate and control the persistence of immune response. The immune checkpoint signaling pathway consists of inhibitory and stimulatory pathways that maintain self-tolerance and assist with the immune response. The efficacy of the immune response is determined by the delicate balance between costimulatory and coinhibitory signals. The signal transduction induced by the interaction of antigenic MHC (Major Histocompatibility Complex)/peptide complexes with T-cell receptor (TCR) is a prerequisite for T cell activation, but it is not sufficient to initiate T cell responses. Further signal transduction of costimulatory molecules is essential for the optimal activation, expansion, and differentiation of the T cells. These molecules are mainly divided into two categories: the immunoglobulin superfamily (IgSF) and the Tumor necrosis factor receptor superfamily (TNFRSF). Tumor necrosis factor receptor superfamily 4 (TNFRSF4) - also known as the OX40 receptor or CD134 - is only expressed upon activation of antigen presenting cells.
TNFRSF4 (a.k.a. CD134, OX40 receptor) encodes a type 1 transmembrane glycoprotein and secondary co-stimulatory immune checkpoint molecule involved in long-term T cell immunity. The tumor necrosis factor receptor superfamily 4 protein is a receptor for OX40L/TNFSF4 and human herpesvirus 6B/HHV-6B.
Background Information: TNFRSF4 Gene Details
TNFRSF4 Aliases: TNF Receptor Superfamily Member 4, Tumor Necrosis Factor Receptor Superfamily Member 4, TAX Transcriptionally-Activated Glycoprotein 1 Receptor, TNFRSF4 , CD134, OX40, and more.
Related Diseases: Immunodeficiency 16 and Acute Graft Versus Host Disease
OX40 Receptor Expression and Activity
The OX40 receptor proteins are only expressed after full activation of CD4, CD8 T cells, and several other lymphoid and non-lymphocytes. OX40 receptors are crucial in maintaining an immune response beyond the first 72 hours - as OX40L binds T cells via OX40 receptors, it improves the survivability of T cells while also increasing cytokine production.
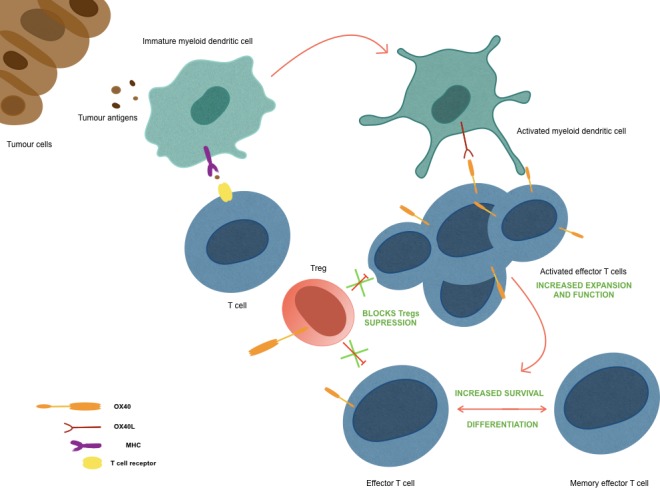
Figure 1: Mechanism of action of CD134/OX40. OX40 counterbalances the inhibition of immune cells (including T lymphocytes CD4+ and CD8+, NK cells and B lymphocytes) while directly stimulating effector T cells.
OX40 Knockout Mouse Model Research
A limited number of studies have been performed with OX40 knockout (KO) mouse models to elucidate its molecular function in vivo. A 2008 study9 in OX40/TNFRSF4 knockout (KO) mice suggested that this receptor plays a role in CD4+ T cell response, as well as in T cell-dependent B cell proliferation and differentiation. It has been shown that OX40 induces expression of proteins with cell-cycle progression (i.e., survivin) and anti-apoptotic (i.e., Bcl-2, Bcl-xl and Bfl-1) properties. Additionally, the OX40 receptor has been shown to activate NF-κB through its interaction with adaptor proteins TRAF2 and TRAF5.
Cyagen AI Knockout Mouse Model eBank features over 16,000 KO/cKO mouse models, as well as 3,000 live mouse strains, delivered in as fast as 2 weeks.
>> View our Tnfrsf4/Ox40 KO Mouse Model
For immunotherapies targeting immune checkpoint blockers (ICB), there is promising evidence related to anti- cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) therapy. Data shows anti-CTLA-4 therapy to yield a selective depletion of regulatory T cells (Treg) in tumor microenvironment (TME) via Fcγreceptor-expressing macrophages. This suggests that OX40-directed antibodies may deplete OX40+ Tregs in TME without reducing effector T cells expressing the receptor. Additional studies showed that OX40 co-stimulation leads to inhibition of FOXP3 gene expression (critical to Treg differentiation) via two independent mechanisms: activating AKT-mammalian target of rapamycin (mTOR) pathway and enhancing the expression of the activator protein 1 transcription factors, BATF and BATF3. Preclinical evidence shows reduction in IL-10 production by tumor-infiltrating Treg cells after treatment with anti-OX40 monoclonal antibody (mAb), allowing maturation of dendritic cells (DC) - likely by downregulation of transcription factor interferon regulatory factor mRNA expression. Therefore, anti-OX40 creates a permissive immune status that leads to myeloid cell accumulation and development of both innate and adaptive immunity - important steps to the anti-tumoral effect of anti-OX40.
Drug Development – Targeting OX40
Costimulatory receptors and pathways may be targeted to regulate T cell signaling and enhance the immune response against tumors. Many drugs that stimulate OX40 signaling have been developed based on in vitro results, including: OX40-specific agonist antibody, OX40L-Fc fusion Protein, dendritic cells (DC) transfected with OX40L mRNA, and tumor cells engineered to express surface OX40L. Table 1, below, features several examples of drugs developed to target OX40:
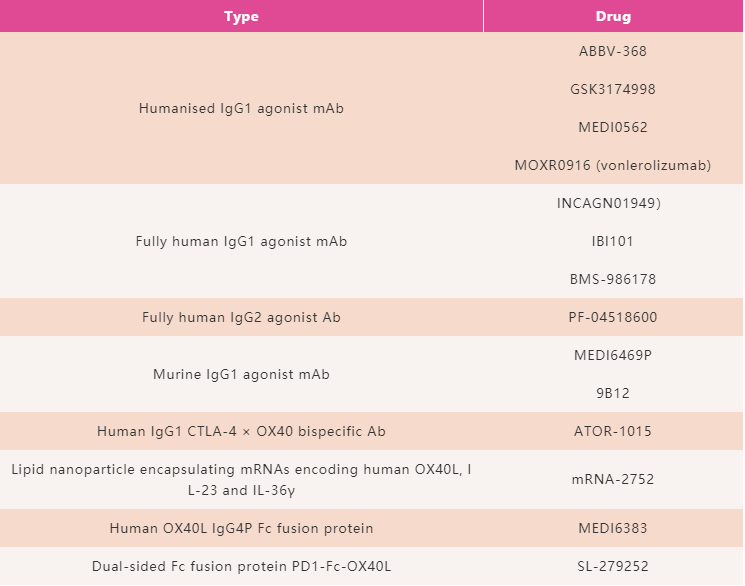
Table 1: OX40-targeted drugs. Shows drugs tested in in vitro studies either in human clinical trials and associated technology type.
Several preclinical cancer models have shown immune cell regulation and anti-tumor activity against OX40. The combined treatment of anti-OX40 and anti-CTLA-4 leads to a significant increase in the proliferation and activity of CD4+ and CD8+ T cells - which translates into better results compared to monotherapy. Although OX40 targeted therapy has shown impressive results in tumor-bearing mice, preliminary clinical data indicate that its efficacy as a monotherapy in humans is not high. However, OX40 stimulation combined with immunotherapy targeting inhibitory receptors - such as anti-programmed cell death 1 (PD-1)/programmed death-ligand 1 (PD-L1) - is a promising strategy. It is hoped that the combination of different anticancer agents and OX40-targeted therapies can be validated in clinical studies soon and bring effective therapies to more patients.
References:
Cyagen now provides hOX40 Mice - Accelerate your Oncology Research
Strain Background: C57BL/6N
Construction Strategy:
The humanized OX40 mouse model (hOX40) is developed by gene knockin (KI) at the mouse OX40 locus and expresses a fully humanized OX40 protein with human extracellular, human transmembrane, and murine intracellular domains.
hOX40 Features:
■ hOX40 expression displays physiological regulation and expression pattern
■ Preservation of the target-ligand interaction
■ Fully functional mouse immune system
■ Lack of expression of the murine target gene, thus avoiding cross-reactivity
Research and Application:
The hOX40 mouse enables the in vivo efficacy assessment and profiling of immuno-oncology agents targeting the human immune checkpoint (ICP) OX40 in fully immunocompetent mice.
Validation Data:
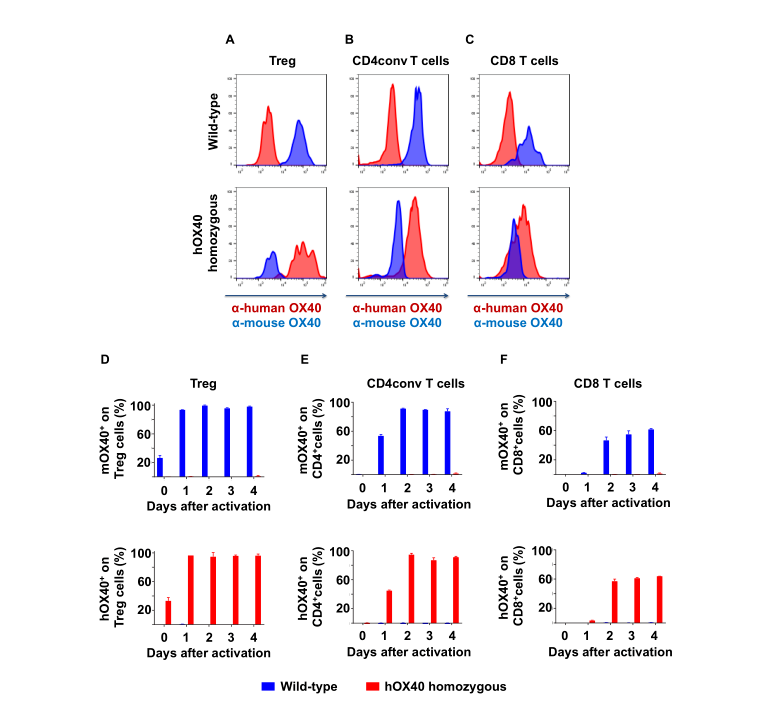
Figure 2. hOX40 expression pattern in hOX40 mice recapitulates mOX40 in WT mice. hOX40 and mOX40 expression on splenocytes activated withαCD3/αCD28 in presence of mIL-2: on (A) Tregs (viable, CD3+CD4+CD25+FoxP3+), (B) conventional CD4+ (viable, CD3+CD4+FoxP3+ ), and (C) CD8+ (viable, CD3+CD8+) T cells at day 3 of culture. Kinetic of hOX40 and mOX40 expression on (D) Treg and (E) conventional CD4 and (F) CD8 T cells.
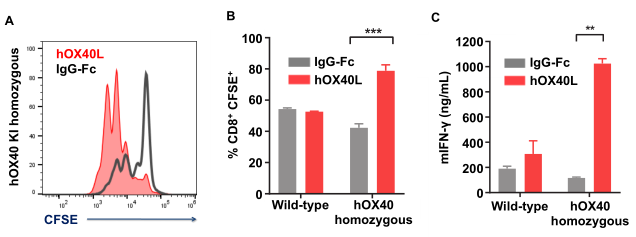
Figure 3. hOX40 is functional on effector T cells ex vivo. Splenic and LN-isolated T cells (CD8+) activated with αCD3/αCD28 and immobilized hOX40L or IgG-Fc. hOX40L treatment significantly promoted the proliferation of T cells (CD8+) and the secretion of IFN in hOX40 mice. (A) Flow chart. (B) Representative histogram and (C) mIFN-γ production measured by ELISA in culture supernatant.
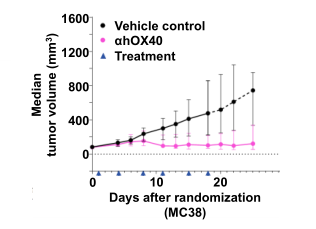
Figure 4. In vivo anti-tumor activity is observed in response to ahOX40. hOX40 mice bearing MC38 tumor were treated with ahOX40 (BMS-986178 analog). The proliferation of tumor was significantly inhibited compared to the control.
Animal models of disease are essential tools for studying the mechanisms of human disease occurrence and development, drug efficacy screenings, and therapeutic assessment (TA). Cyagen Drug Screening and Assessment Mouse Model Platform provides various tumor mouse models, including BALB/c nude, NOD scid, and immunodeficient mice - like C-NKG, immune checkpoint (ICP) humanized mice, syngeneic model, human tumor cell line xenotransplantation (CDX) models – alongside our genetically engineered murine model and phenotype analysis services.
If you need immediate assistance, please call 86 20-31601779 or email service-apac@cyagen.com.
We will respond to you in 1-2 business days.