
Due to the significant genetic similarity between humans and mice, transgenic mice are important tools for scientists researching heritable traits and diseases in human populations. In traditional transgenics, the human gene (packaged in a transgenic construct) can be added to the mouse genome with a simple pronuclear injection into the male pronucleus. Transgenic model organisms are helpful in research for studying human diseases and genetic disorders, such as heart disease, obesity, Parkinson’s disease, and substance abuse disorders – exemplified by countless research publications and therapeutic progress enabled by transgenic models.
Watch our newest video to learn about the steps involved in pronuclear injection (PNI)-mediated generation of transgenic mouse models, which are used by scientists to study a wide range of human diseases.
Scientists have already created many amazing transgenic animals, some of which are even commercially available. GloFish - introduced to the US market in 2003 - are one of the earliest examples of transgenic pets. These zebrafish have been genetically engineered to contain genes from other organisms, such as jellyfish or sea anemones, that result in expression of fluorescent proteins. Additional species of fish have been developed to express a range of colors, using genes extracted from a variety of organisms which naturally produce fluorescent proteins. These fluorescent transgenic pets have a strong basis in development of model organisms for research - the ability to express fluorescent proteins under certain conditions has been utilized in many genetic engineering studies.
A Transgenic Organism is an individual of a species that contains a gene from another individual of the same species or from another species. These genes from another species are called transgenes. Transgenesis is the process used by scientists to introduce a gene from one animal to another: This process has three main steps:
Before we go over the steps involved in pronuclear injections (PNIs), let us first cover the biological basics of the process. As you may know, all female mammals are born with all the reproductive cells inside of them that they will produce for their entire lives - these are called oocytes. The fertilization of oocytes offer scientists many opportunities to take advantage of important cellular mechanisms. Within 24 hours of fertilization the fertilized oocytes will have developed into zygotes, at which point two pronuclei appear inside of the embryo.
Let us move on to the steps of a pronuclear injection (PNI) and what happens inside the mouse genome as these steps progress:
To determine which offspring contain the gene, snippets of their tails are tested using PCR genotyping. The mice that test positive for the transgene can be mated with other mice to establish lines of transgenic mice.
To obtain transgenic mice via pronuclear injection (PNI), scientists must inject many zygotes with the transgene before implanting them into a surrogate mother. Although many of them will die, each one of them possesses the potential to turn into its own transgenic mouse. In a Traditional pronuclear injection (PNI), each of these zygotes will undergo a totally random integration of the transgene as it finds its way into the animal's genome. As noted in step 4 (above) of the PNI-based Transgenic mouse model generation process, injection of the male pronucleus results in random integration of the transgene construct. Scientists think this may occur as DNA repair enzymes seek out and repair broken ends of DNA. However, the exact mechanisms for random integration have not yet been determined. This randomness includes the location of integration, how many different sites undergo integration, and how many copies of the gene are integrated into each site. These three major factors will lead to great varieties in the expression level of the offspring.
As you can see, using standard pronuclear injection (PNI) is a simple way to get transgenic mice, but it leads to big variations in levels of gene expression in the offspring. For this reason, scientists have developed other more efficient methods of creating transgenic mice, such as PiggyBac transgenesis, which we will talk about in our next video.
In addition, the rapidly growing capabilities of CRISPR- and embryonic stem (ES) cell-mediated technologies, such as our proprietary TurboKnockout® Gene Targeting services, have enabled the production of transgenic, large fragment knock-in (LFKI), and humanized mouse models.
Cyagen provides comprehensive model generation services for all your transgenic mouse, mouse embryo, and rat model needs, including:
The table below offers a brief overview of the general capabilities provided by our leading service options for generating transgenic rodent models for research.
Comparison of Transgenic Model Generation Techniques and Capabilities
|
|
Regular Transgenic |
PiggyBac Transgenic |
Rosa26 Targeted Transgenic |
|
Integration |
Random, multicopy integration |
Random, single copy per integration site |
Single copy transgene targeted to Rosa26 safe harbor locus |
|
Vector construction |
Transgenic plasmid or BAC |
Transgenic plasmid or BAC |
Targeting vector + gRNAs |
|
Expression pattern |
Variable expression in founders |
More consistent expression in founders
|
Most consistent expression
|
|
Endogenous effects |
Can disrupt endogenous gene expression |
Less likely to disrupt endogenous gene expression |
Safe harbor site (SHS) does not disrupt endogenous gene expression |
|
Zygosity |
Hemizygous |
Hemizygous |
Options: Heterozygous and Homozygous |
|
Turnaround |
2-5 months |
2-5 months |
6-9 months (F1) |
|
Species |
Mouse, mouse embryos, rats |
||
|
Donor background |
Mouse strains: C57BL/6, FVB Rat strains: Sprague-Dawley (SD), Long Evans Note: Other strains available upon request. |
||
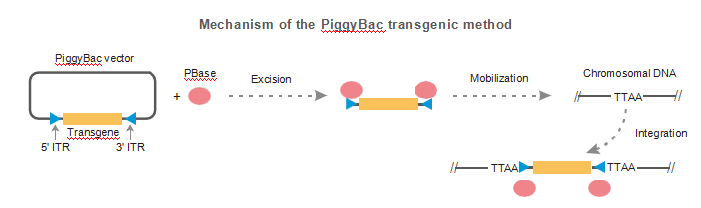
Our proprietary PiggyBac transgenic method has the following advantages over other transgenic approaches:
●Single-copy integration: Avoids potential gene silencing from multiple copies per integration site
●Defined region of integration: No loss of transgene sequence (TTAA, transcription unit)
●Reliability: More consistent expression pattern compared to plasmid-based transgenics
●Economical: Cost and turnaround time comparable to plasmid-based transgenics
Cyagen has successfully generated large fragment knock-in mouse models using TurboKnockout® or CRISPR, across both endogenous gene loci and Rosa26 loci. We provide a complete range of services, from generation of the engineered parental ES cell line through delivery of research-ready custom mouse models.
Using data from thousands of knock-in mouse model projects completed by our team, we have collected new information demonstrating how our gene editing technologies push the boundaries of modifying large genomic regions. Below, we have outlined Cyagen’s large fragment knock-in (LFKI) capabilities across our gene editing technologies.
Comparison of TurboKnockout® and CRISPR Methods for LFKI Model Generation
|
|
TurboKnockout® |
CRISPR/Cas9 Gene Editing |
|
Approach |
Homologous recombination in ESC by our proprietary TurboKnockout® technology |
CRISPR/Cas9 nuclease mediated gene targeting by pronuclear injection |
|
Applications |
Conditional knockout |
Conditional knockout |
|
Knock-in (KI) fragment size limits |
~20 kb per round of gene targeting |
Endogenous: ~15 kb |
|
Conditional Knockout (cKO) |
Single target: ~7 kb |
Donor vector: ~7 kb |
|
Donor backgrounds |
Mouse strains: C57BL/6, BALB/c |
Mouse strains: C57BL/6, FVB |
|
Turnaround time |
6-8 months |
5-7 months |
From strategy design through to delivery of research-ready custom mouse models, Cyagen offers complete outsourcing for all your animal model needs. Cyagen’s gene editing services are unparalleled in efficiency of developing rodent models with a guaranteed genotype. We even offer price matching to help ensure researchers get the best deal for their study.
Contact us to perform your entire transgenic project - from initial strategy design and DNA vector construction, all the way through breeding – we deliver research-ready transgenic rodent models for guaranteed results.
We will respond to you in 1-2 business days.