
In most experiments, scientists will try to minimize the number of independent variables so as not to confuse the results of an experiment. Due to the varying level of gene expression achieved by traditional pronuclear injection (PNI), using the offspring from a transgenic mouse created via PNI may not be the best option.
Using standard pronuclear injection (PNI) is a simple way to get transgenic mice, but it leads to big variations in levels of gene expression in the offspring. For this reason, scientists have developed other more efficient methods of creating transgenic mice, such as the PiggyBAC transposon. For over a decade, scientists have used the PiggyBAC transposon to generate transgenic mice with single copy integration, targeted into areas of open chromatin.
Watch the video to learn how using PiggyBAC to generate transgenic mice leads to high and consistent expression of the transgene across all F1 individuals.
In the last video (Pronuclear Injection-based Transgenic Mouse Model Generation) we talked about what transgenic mice are and how they are developed by scientists using traditional pronuclear injection (PNI). However, there are some limitations to using the pronuclear injection method to create a transgenic animal.
In a traditional pronuclear injection (PNI) transgenics, multiple copies of the transgene may be inserted as the foreign DNA integrates into the target genome. Contrary to the common belief that these tandem repeats increase the expression of a gene, multiple copies of a gene can lead to a high variety of expression depending on the region of integration. At some loci, such tandem integration may increase the expression of the target gene, while at others, it may silence the expression of the gene itself.
To obtain transgenic mice via pronuclear injection (PNI), scientists must inject many zygotes with the transgene before implanting them into a surrogate mother - each one of them possesses the potential to turn into its own transgenic mouse. In a traditional pronuclear injection (PNI), random integration of the transgene construct occurs as it finds its way into the animal's genome. This randomness includes the location of integration, how many different sites undergo integration, and how many copies of the gene are integrated into each site. These three facets of random integration results in big varieties of the gene expression level in the offspring. Since transgene integration is a random event in PNI-mediated generation of transgenic mice, one of the main disadvantages of the PNI method is that the transgene cannot be directed to a targeted chromosomal location.
The different locations that the transgene may integrate into on the chromosome may play a part in explaining the variability of the gene expression. Just like random integration means random number of copies, it means random location as well. Chromatin structure plays a key role in regulating gene expression by allowing DNA accessibility to transcriptional machinery and transcription factors. If the DNA integrates into an area of closed chromatin, it will be harder for the transcriptional machinery to access that section of DNA and result in a lower frequency of expression compared to DNA integration into an open chromatin region (OCR).
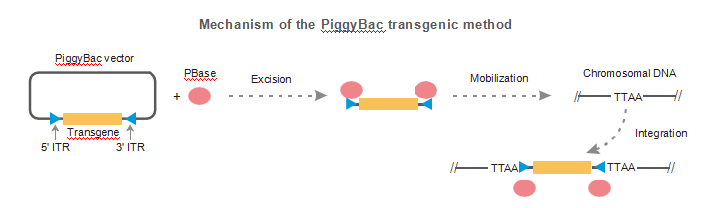
The PiggyBAC method uses transposons to integrate a single copy of the transgene into animals which leads to higher, more consistent expression of those genes. We will briefly introduce what a transposon is and how it works before moving onto the specifics of PiggyBAC transgenics.
A transposon is a DNA segment which moves from one area of a gene to another. For this reason, transposons are referred to as jumper genes. The process by which a transposon moves from one area to another is known as transposition.
There are two different classes of transposons which undergo transposition in distinctly different ways. Type one transposons are known as retrotransposons. These retrotransposons convert themselves to RNA using transcription and then back to DNA using reverse transcription - once converted back into DNA they are then inserted into the new genome. Alternatively, PiggyBac is a DNA transposon. In this kind of transposition, DNA moves directly from one area of the gene to another in the same way that one might ‘cut’ and ‘paste’ text on a computer.
The PiggyBAC method uses transposase enzymes to excise the target DNA from the host genome, just like all DNA transposons. Transposase enzymes recognize inverted terminal repeat (ITR) sequences which are on either side of the target DNA. The short 13 base pair (bp) sequences are perfect reverse complements of each other and are also known as inverted terminal repeat domains (TRD's).
The procedure for completing a PiggyBAC transgenic mouse is identical to that of a regular transgenic mouse except for how the vector is developed and the way that it is injected.
In traditional pronuclear injection (PNI), you inject a single transgenic construct. However, in a PiggyBAC transgenic project, a co-injection is performed - containing both the PiggyBAC transposon (with the transgene) and transposase enzymes (which will recognize and cut the ITR sequences). In most cases, a vector that expresses the transposase enzyme through its DNA is used, because the enzyme is very costly to obtain in comparison.
The only other difference between these two processes occurs inside of the cell after the PiggyBAC transposon plasmid with the transgene has been injected into the male pronucleus of the zygote. After the injection of the PiggyBAC transposon into the male pronucleus, transposase enzymes find their way to the inverted terminal repeat (ITR) sequences on the gene. Next, the transposon will dimerize to form a complex called a transpososome, which excises the transgenic DNA (including ITRs) from the PiggyBAC transposon plasmid. The transpososome is then guided through the nucleus toward certain areas in the genome which are associated with stability and high expression.
The transpososome is guided toward areas with large amounts of TTAA sequences, which associate closely with open chromatin. Chromatin structure plays a key role in regulating gene expression by allowing DNA accessibility to transcriptional machinery and transcription factors. Areas of open chromatin are areas which have high rates of transcription. Thus, integration of transgenes into these regions has led to higher expression of the genes.
Given that the gene was cut at the ITR’s, only a single copy will integrate into the genome per TTAA site. This difference leads to a more consistent expression of the gene, while the integration into TTAA sites associated with open chromatin leads to higher expression.
PiggyBAC is a great option for creating transgenic mouse and rat models with high expression and consistent results. With a comparable timeline to standard pronuclear injection (PNI), PiggyBAC transgenic projects provide single-copy integration of your transgene sequence for consistent expression.
In addition, the rapidly growing capabilities of CRISPR- and embryonic stem (ES) cell-mediated technologies, such as our proprietary TurboKnockout® Gene Targeting services, have enabled the production of transgenic, large fragment knock-in (LFKI), and humanized mouse models.
Our proprietary PiggyBac transgenic method has the following advantages over other transgenic approaches:
●Single-copy integration: Avoids potential gene silencing from multiple copies per integration site
●Defined region of integration: No loss of transgene sequence (TTAA, transcription unit)
●Reliability: More consistent expression pattern compared to plasmid-based transgenics
●Economical: Cost and turnaround time comparable to plasmid-based transgenics
Cyagen provides comprehensive model generation services for all your transgenic mouse, mouse embryo, and rat model needs, including:
The table below offers a brief overview of the general capabilities provided by our leading service options for generating transgenic rodent models for research.
Comparison of Transgenic Model Generation Techniques and Capabilities
|
|
Regular Transgenic |
PiggyBac Transgenic |
Rosa26 Targeted Transgenic |
|
Integration |
Random, multicopy integration |
Random, single copy per integration site |
Single copy transgene targeted to Rosa26 safe harbor locus |
|
Vector construction |
Transgenic plasmid or BAC |
Transgenic plasmid or BAC |
Targeting plasmid |
|
Expression pattern |
Variable expression in founders |
More consistent expression in founders
|
Most consistent expression
|
|
Endogenous effects |
Can disrupt endogenous gene expression |
Less likely to disrupt endogenous gene expression |
Safe harbor site (SHS) does not disrupt endogenous gene expression |
|
Zygosity |
Hemizygous |
Hemizygous |
Options: Heterozygous and Homozygous |
|
Turnaround |
2-5 months |
2-5 months |
6-9 months (F1) |
|
Species |
Mouse, mouse embryos, rats |
||
|
Donor background |
Mouse strains: C57BL/6, FVB Rat strains: Sprague-Dawley (SD), Long Evans Note: Other strains available upon request. |
||
From strategy design through to delivery of research-ready custom mouse models, Cyagen offers complete outsourcing for all your animal model needs. Cyagen’s gene editing services are unparalleled in efficiency of developing rodent models with a guaranteed genotype. We even offer price matching to help ensure researchers get the best deal for their study.
Contact us to perform your entire transgenic project - from initial strategy design and DNA vector construction, all the way through breeding – we deliver research-ready transgenic rodent models for guaranteed results.
We will respond to you in 1-2 business days.