
Recently, the U.S. Food and Drug Administration (FDA) has approved Carvykti, a chimeric antigen receptor (CAR) T-cell therapy product jointly developed by Legend Biotech and Johnson & Johnson, for the treatment of relapsed/refractory multiple myeloma. As the world's second CAR-T product for multiple myeloma, Abecma, Carvykti’s competitor, was jointly developed by BMS and Bluebird Bio. Clinical data has shown that the objective response rate (ORR) and stringent complete response (sCR) of Carvykti are 97.9% and 78.4% respectively [1]; the ORR of Abecma is 72%, and the sCR is 28% [2]. Statistically, the efficacy of Carvykti has a significant advantage. Carvykti took nearly 4 years from its first clinical trial approval to its final launch, and preclinical research also took significant time and effort. The efficacy and safety evaluation of preclinical drugs is crucial for investigational new drug (IND) application. This article will introduce the selection of pharmacological models centering on preclinical studies of CAR T-cell therapy.
In Vitro Model
The mechanism of CAR T-cells is specifically recognizing tumor target antigens through the single-chain fragment variable (scFv) antibody of CAR molecules and then killing them. Therefore, genetically constructed cells expressing tumor antigens can be used to examine the cytotoxicity of CAR-T therapy. For example, the 293T cells are genetically modified ex vivo by transduction with lentiviral vectors to overexpress CD19, of which the expression will be considered as a simulation of CD19-tumor cells. After the examination of the antigen positive rate, the CD19-293T cells will be used for further experiments.
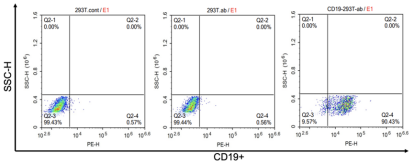
Figure 1. Detection of CD19 antigen positive rate of CD19-293T cells. (Source: Cyagen)
In addition to genetically constructed tumor cell models, tumor cell lines that naturally express antigens have also been used to test the cytotoxicity of CAR T-cells. Several ways can be used to examine the cytotoxicity of CAR T-cells, such as flow cytometry, luciferase enzymatic method, chromium release test, etc. Accordingly, we list some cell models that are available for cytotoxicity testing, as well as examples of their applications.
For example, Novartis used the luciferase assay in the BCMA-targeted CAR-T study, which was published in 2018 [3]. Luciferase catalyzes the oxidation of luciferin, during which bioluminescence is emitted. The researchers introduced the firefly luciferase (Luc) gene into human multiple myeloma cells KMS-11 to construct KMS-11-luc cells. Then, CAR T-cells and KMS-11-luc cells were co-incubated at different effector-target (E:T) ratios for 20 hours, and the fluorescence intensity of the cell mixture was detected, which could represent the quantity of remaining target cells.
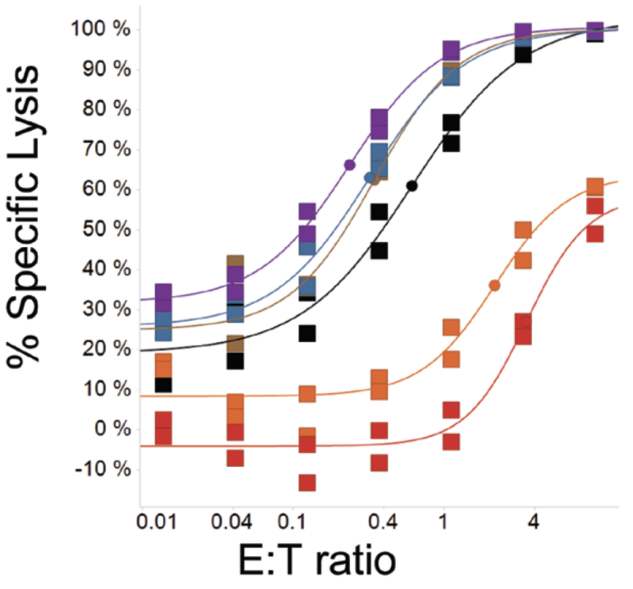
Figure 2. Tumor cell lysis curves using the luciferase assay [3].
In a 2015 joint study by the University of Pennsylvania and Novartis, the chromium release assay was used to detect the cytotoxicity of CAR T-cells [4]. First, the isotope 51Cr was used to label U87 glioma cells, and then CAR T-cells were co-incubated with tumor cells at different effector-target (E:T) ratios. After the tumor cells were lysed, 51Cr was released from the cells to the supernatant, and the content of 51Cr in the supernatant was detected, which could represent the number of lysed tumor cells.
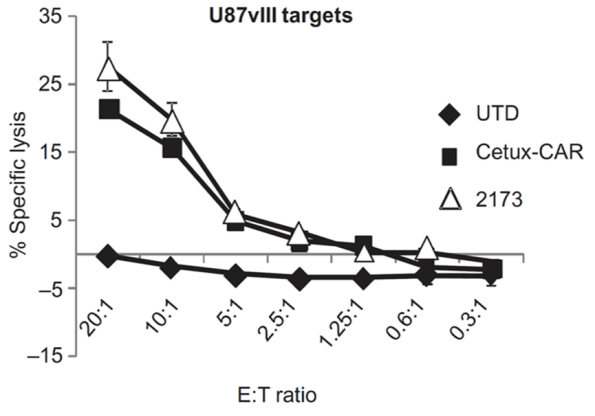
Figure 3. Tumor cell lysis curves using the chromium release assay [4].
In addition to tumor cell models, Jurkat reporter cells can also be used in place of T cells isolated and activated from PBMCs for early evaluation experiments. Jurkat cells are an immortalized cell line of human T lymphocytes, originally established in the late 1970s from the peripheral blood of a 14-year-old boy with T-cell leukemia. In CAR-T in vitro experiments, Jurkat cells are often used for the study of T-cell signaling.
In a 2019 article by Memorial Sloan-Kettering Cancer Center, in order to investigate whether CAR T-cells are antigen-dependent, the red fluorescent protein (RFP) gene was delivered to the downstream region of CD3ζ signaling in Jurkat cells, and green fluorescent protein (GFP) was expressed on the CAR molecule to construct CAR/GFP-modified Jurkat-RFP reporter cells [5]. Green fluorescence represents the successful transduction of the CAR molecule, while red fluorescence represents the successful CAR signaling. CAR/GFP-modified Jurkat-RFP reporter cells were co-incubated with antigen expressing target cells and non-expressing control cells, respectively. In the control group that did not express antigen, the presence of both green and red fluorescence indicated that this class of CAR T-cells were Antigen-independent, that is, not ideal CAR T-cells.
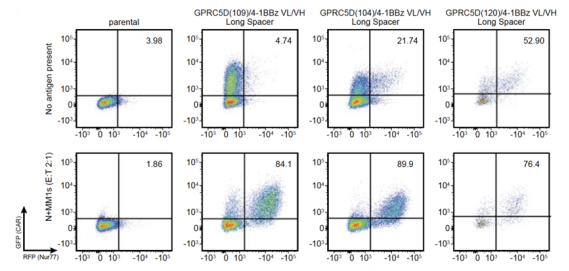
Figure 4. Fluorescence expression of CAR/GFP-modified Jurkat-RFP reporter cells detected by flow cytometry (ordinate: green fluorescence; abscissa: red fluorescence) [5].
In Vivo Model
In the preclinical study of CAR-T therapy, the evaluation of the efficacy of animal tumor models is particularly important. Due to a high similarity between the mouse genome and the human genome, and the low cost of constructing mouse models, mice have become one of the best experimental models for studying human diseases. Immunodeficient mice have weak rejection of grafts and are very suitable for the construction of animal tumor models. At present, it is recognized internationally that the mice with the highest degree of immunodeficiency are those with Prkdc and Il2rg gene defects. Mutations in the two genes completely inactivated T cells, B cells, and NK cells in the mice. For example, in C-NKG severe immunodeficiency mouse (NOD/Shi-Prkdcscid Il2rgem1/Cgen) independently developed by Cyagen, the Il2rg gene was knocked out in the NOD/Shi-scid background strain. The C-NKG mouse model has the characteristics of decreased complement activity, weak phagocytosis of human cells by macrophages, decreased dendritic cell function, and no leakage of T cells and B cells. It is suitable for the development of: CDX and PDX models, humanized mouse models of the immune system, GvHD models, and studies related to tumor immunity.
In addition to severely immunodeficient mice, nude mice, severe combined immune-deficiency mice (SCID) mice, and NOD/SCID mice can also be used for tumor model construction. Nude mice were the first immunocompromised mice discovered in the UK in the 1960s. Severe combined immune-deficiency mice (SCID) have a Prkdc mutation, which is characterized by loss of T cells and B cells. NOD/SCID mice were generated by crosses between non-obese diabetic (NOD) mice and SCID mice, and the degree of immunodeficiency was further enhanced. With the continuous development and progress of biotechnology, immunodeficient animals are constantly being optimized. Selecting suitable immunodeficient animals can achieve better results in experiments and help research and development in related fields.
The Cell line-Derived Xenograft (CDX) is a model in which tumor cell lines are transplanted into homogenous or xenogeneic animals to form tumors. Tumors formed after tumor cell line transplantation retain most of the biological characteristics of the primary tumor, with high tumor formation efficiency and short experimental period, so they are very suitable for early efficacy evaluation of tumor drugs. In studies of CAR-T therapy, tumor cells containing luciferase genes are used to construct CDX models to monitor the growth of tumor cells and anti-tumor activity of CAR T-cells by fluorescence.
The Patient-Derived Xenograft (PDX) is a model established by directly transplanting fresh tumor tissue from patients into immunodeficient mice. Tumor tissue grown in mice and passaged 2-3 times can better represent the parental tumor traits and maintain tumor heterogeneity. Compared with CDX, the biggest advantage of PDX is that it retains the heterogeneity and histological properties of parental tumors, such as cell morphology, vascular system, stroma morphology, etc., simulating the in vivo environment of tumorigenesis.
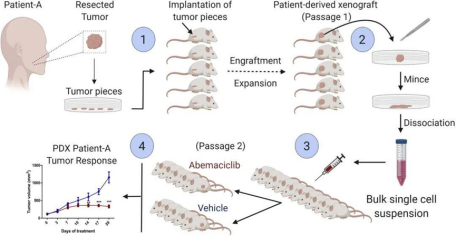
Figure 5. PDX construction process [6].
A syngeneic model is a model in which tumor cell lines of the same background are inoculated into immunocompetent inbred mice. The recipient mice have a complete murine immune system with complete immune activity, and the immune system is compatible with the allograft tumor tissue, which can maximize the simulation of the real conditions of the tumor microenvironment. The disadvantage is that the transplanted mouse tissue may not fully represent the complexity of human tumors in the clinical setting.
Human immune system-engrafted models (HIS) are generated by xenotransplanting human immune cells or tissues and/or their progenitor cells into immunodeficient mice. These mice can more effectively simulate the human immune response, providing an excellent model for the transformation of tumor immunity, autoimmune diseases, and human-specific infectious diseases such as AIDS, the acquired immune deficiency syndrome caused by HIV, the human immunodeficiency virus.
Similar to the principle of constructing luciferase-expressing tumor cells in the in vitro model, for the in vivo model, the oxidative luminescence properties of luciferin are also used. The size and distribution of the tumor in vivo are monitored by an in vivo imaging system and quantified by the fluorescence mean intensity (FMI). For example, when constructing the CDX model of acute lymphoblastic leukemia, the luciferase gene can be delivered to the human acute lymphoblastic leukemia cell Nalm6 to construct Luci-Nalm6 cells. Different doses of Luci-Nalm6 are inoculated into C-NKG and NOG mice, and the in vivo imaging on day 21 is shown in the figure below:
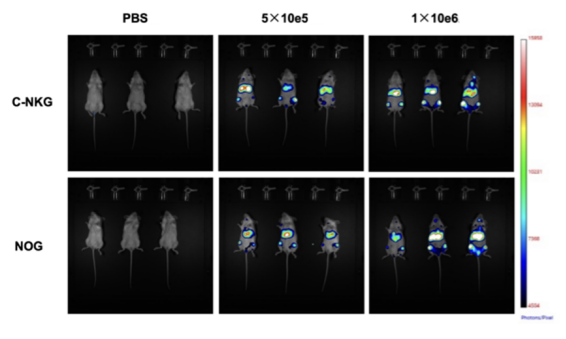

Figure 6. In vivo imaging of tumor growth in luci-NalM6 cell xenogeneic mouse tumor model (above: In vivo imaging on day 21; below: fluorescence mean intensity). (Source: Cyagen)
Why Choose Cyagen?
In the preclinical research of CAR-T therapies, the selection of cell models, animal models, and modeling strategy is crucial. Clarifying the purpose of the research and establishing effective experimental models and protocols will make experiments more efficient. Cyagen provides one-stop services to help CAR-T and cell therapy research and development, including antibody screening, stable cell lines construction, development of cell/animal models, and drug efficacy evaluation.
We will respond to you in 1-2 business days.