
The human S100A9 gene encodes the S100 calcium-binding protein A9 (S100A9). In mammals, S100A8 and S100A9 proteins form a heterodimer known as calprotectin. Calprotectin is involved in the inflammatory process - it is known to be present as a soluble protein in the cytosol of neutrophil granulocytes, and is found in lower concentrations among in monocytes, macrophages, and squamous epithelial cells. The following study has identified S100a8/a9 as key mediators of early myocardial ischemia-reperfusion (MI/R) injury that may serve as potential therapeutic intervention targets.
Title: S100a8/a9 Signaling Causes Mitochondrial Dysfunction and Cardiomyocytes Death in Response Ischemic/Reperfusion Injury
Journal: Circulation
Impact factor (IF): 23.054
Publication Date: 2019.06.21
Selected product: S100a9 gene knockout (KO) mice
Step 1 Dynamic transcriptomic analysis
Researchers performed time series transcriptomic analysis to learn the dynamic pathological alterations of mouse hearts at different stages of MI/R. Dynamic transcriptomic analysis screened and identified S100a8/a9, an inflammatory molecule, as the key mediator of MI/R injury. In the early reperfusion stage, S100a8/a9 was identified as the most significant up-regulated gene – its expression was confirmed via immunochemistry and reverse-transcriptase PCR in mice hearts after I/R.
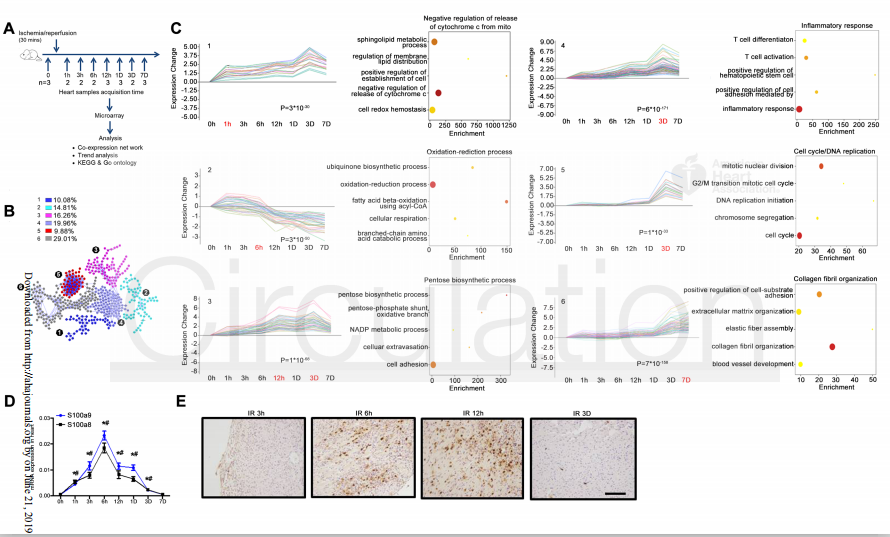
Figure 1. Dynamic transcriptomic analysis on mouse hearts at different stages of MI/R, and identified S100a8/a9 is a dynamically regulated key mediator of MI/R injury. Mice were subjected to myocardial infarction (MI), followed by different lengths of reperfusion (R) periods.
Step 2 Function loss/gain experiment
Researchers used both the loss- and gain-of-function approaches to study the role of S100a8/a9 in MI/R injury. For these approaches, S100a9 gene knockout (KO) mice and S100a9 transgenic (TG) overexpression mice models were implemented for comparison with wild-type (WT) mice. S100a9 gene knockout (KO) was shown to significantly reduce cardiomyocyte (CM) death, decrease infarct size, and improve cardiac function, while S100a9 overexpression aggravates MI/R injury. These results indicate that S100a8/a9 is critical for the sequelae of I/R-induced myocardial death, adverse cardiac remodeling, and heart failure.
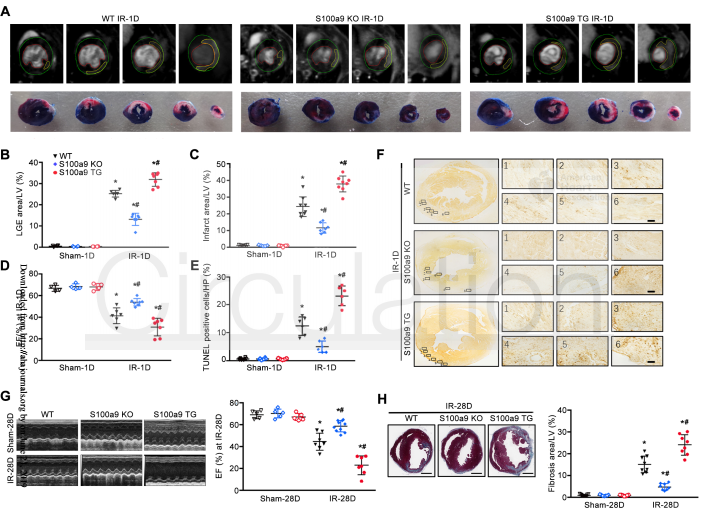
Figure 2. S100a8/ A9 promotes cardiomyocyte death and heart failure during MI/R
Step 3 Transcriptomic and functional experiments
To further explore the mechanism of action of S100a8/a9, the researchers conducted transcriptomic and functional experiments. The results show that S100a8/a9 leads to cardiac mitochondrial respiratory dysfunction. S100a8/a9 down-regulates NDUF gene expression with subsequent mitochondrial complex I inhibition via Toll-like receptor 4(TLR4)/Erk–mediated Pparg coactivator 1 alpha (PGC-1α)/nuclear respiratory factor 1 (NRF1) signaling suppression. S100a9 neutralizing antibody is shown to significantly reduce MI/R injury and improve cardiac function.
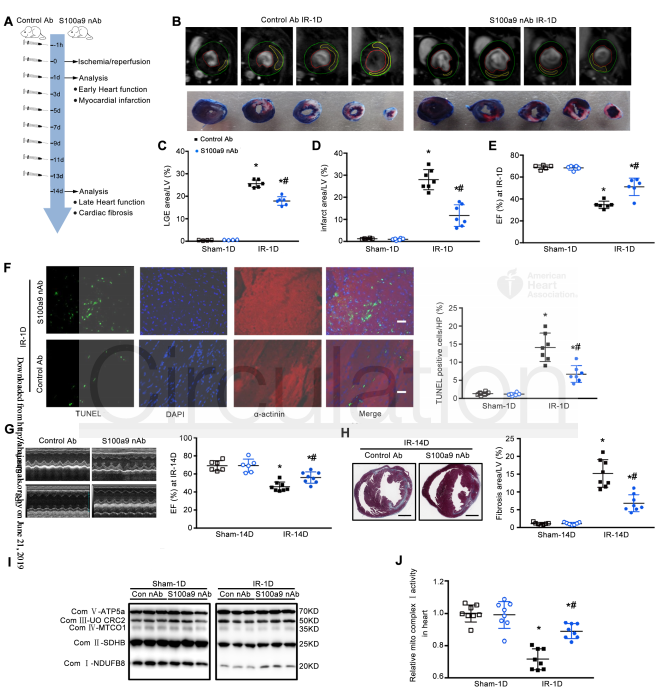
Figure 3. Blocking S100a9 inhibits MI/R injury
Step 4 Clinical research verification
In order to examine the clinical correlation between the elevated S100a8/a9 and MI/R injury, the researchers evaluated the dynamic changes of serum S100a8/a9 levels after percutaneous coronary intervention (PCI) in acute myocardial infarction (AMI) patients and its predictive value for major cardiovascular events (MACEs). It was found that the serum S100a8/a9 level was significantly increased on the first day post-PCI in AMI patients, indicating that the increase in S100a8/a9 level was related to the incidence of major adverse cardiovascular events.
This study demonstrates for the first time that S100a8/a9 directly promotes CM death after I/R by a novel intracellular mechanism which involves mitochondrial complex I dysfunction. Clinical research has shown that increased levels of S100a8/a9 after PCI are associated with poor prognosis in patients with AMI. Overall, these findings indicate S100a8/a9 to be a key regulator of CM death in early MI/R injury, and that S100a8/a9-initiated signaling may serve as both a new therapeutic intervention target and a useful prognostic biomarker for MI/R injury.
We will respond to you in 1-2 business days.