

If you want to effectively achieve gene up-regulation or knockdown, constructing an overexpression stable cell line through lentiviral transfection will be your best choice, which can achieve long-term and stable regulatory effects. Read on to learn how to make a lentiviral overexpression vector!
Lentiviral vectors are a promising tool for both in vivo and ex vivo gene therapy [1][2]. These vectors can be used for therapeutic strategies relying on both transgene expression and gene correction [3]. In addition, lentiviral gene vectors are extensively used in basic biomedical research to deliver genes for the expression of proteins and RNAs, e.g. shRNAs for gene knockdown. Lentiviral gene vectors are also being investigated as vaccines for immunization [4].
Lentiviral Vector Design
First, we need to determine the target gene sequence, that is, the transcript of the target gene. Then select the appropriate lentiviral vector backbone and corresponding genetic elements according to the actual needs of the experiment. The following introduces the characteristics of different elements through a conventional lentiviral vector backbone (Figure 1).
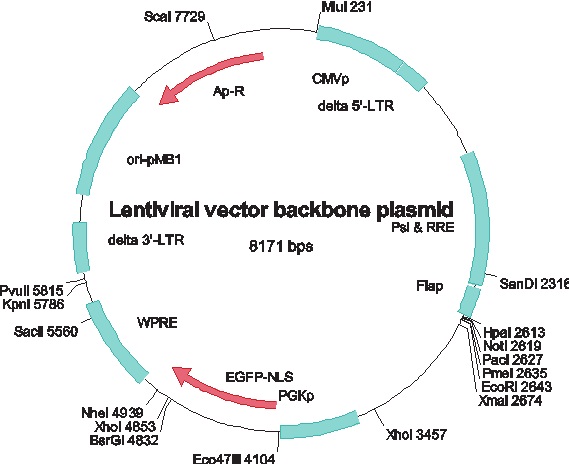
Figure 1. A typical lentiviral vector backbone plasmid. CMVp – the immediate early CMV promoter; delta 5’-LTR and delta 3’-LTR – the long terminal repeats with some deletions; Psi & RRE – the packaging sequence ψ and the Rev Response Element (RRE); PGKp – the mouse phosphoglycerol kinase promoter; EGFP-NLS – the gene for nuclear targeted enhanced GFP protein; ori-pMB1 – the multicopy origin of replication originating from the wild type plasmid pMB1; Ap-R – the ampicillin resistance marker, the gene for β-lactamase[4].
Promoter Sequence
The promoter is located upstream of the 5'-end of the gene, which can activate RNA polymerase and enable it to accurately bind to DNA and initiate transcription. In routine overexpression experiments, we can consider some commonly used promoters, such as CMV, EF1α, and CAG promoters. These promoters have characteristics of both strong driving activity and broad-spectrum application. CMV is particularly suitable for gene expression in somatic or adherent cells such as tumor cells, muscles, and liver; EF1α promoter is particularly suitable for gene expression in stem cells, primary cells, hematopoietic cells, etc.; and CAG promoter is particularly suitable for expression in immune cells and somatic cells. If you need to ensure tissue specificity, you can use tissue-specific promoters to better drive the expression of target genes in specific tissues or cells. For example, CaMKIIa and hSyn can be specifically expressed in neurons; Afp, Alb, and Tbg can be specifically expressed in liver cells (Table 1).
|
Constitutive |
Features |
Specificity |
Features |
|
CAG |
Strong driving activity,Widely expressed, Longer sequence |
CaMKIIa |
Neuron specificity, High expression |
|
CMV |
Strong driving activity,Widely expressed, Commonly used |
hSyn |
Neuron specificity, High expression |
|
EF1α |
Widely expressed |
Afp |
For hepatocytes |
Table 1. Commonly used promoters of lentiviral vectors (parts)
Fluorescence Labeling & Selectable Markers
Fluorescent tags are used to detect the expression efficiency of the vector. Commonly used fluorescent labels are GFP, EGFP, mCherry, etc. (Table 2), which can be selected according to actual experimental conditions. Luciferase proteins catalyze emission of bioluminescence from the enzymatic conversion of a supplemented cofactor. It should be noted that if luciferase is used in the experiment, it is best not to choose EGFP. When the two are placed in the same lentiviral vector, either Luciferase or EGFP can't luminesce or fluoresce normally. Selection markers are used to screen out non-resistant, untransfected cells. There are unique resistance cassettes available depending on the eukaryotic or prokaryotic cells used. The eukaryotic resistance markers include Puromycin, Neomycin, Hygromycin, Blasticidin, etc., and the prokaryotic resistance markers include Ampicillin and Kanamycin, which are used for bacterial culture plasmid amplification.
|
Fluorescent protein |
Fluorescence characteristics |
|
GFP |
Green fluorescence |
|
EGFP |
Enhanced type, 6 times of EGFP |
|
copGFP |
1.3 times of EGFP |
|
mNeonGreen |
Yellow-green fluorescence |
|
mCherry |
Red fluorescence |
|
dTomato |
Red fluorescence |
Table 2. Commonly used fluorescent labels
Long Terminal Repeat (LTR): 5' LTR and 3' LTR
The long terminal repeat (LTR) is the control center for gene expression. The integrated provirus has two LTRs, and the 5' LTR normally acts as an RNA pol II promoter. The 3' LTR is not normally functional as a promoter, although it has exactly the same sequence arrangement as the 5' LTR. Instead, the 3' LTR acts in transcription termination and polyadenylation. The sequence between 5' LTR and 3' LTR is the region where the target gene is inserted. Inserting target genes exceeding 9 Kb will reduce the titer of the lentivirus. It is not recommended to construct a reverse expression vector, as reverse expression will affect the virus titer. Lastly, it is best not to add polyA between 5' LTR and 3' LTR. Since HIV is a retrovirus, adding polyA will affect viral transcription.
There will be other elements in the lentiviral vector that serve to improve the packaging efficiency, titer, and target gene expression efficiency, such as Ψ, RRE, CPPT, etc. (Table 3).
|
Element |
Purpose |
|
Psi (Ψ) |
RNA target site for packaging by Nucleocapsid. |
|
The HIV-1 Rev response element (RRE) |
The Rev-RRE oligomeric complex mediates the export of messages from the nucleus to the cytoplasm, where they are translated to produce essential viral proteins and/or packaged as genomes for new virions. |
|
cPPT/CTS |
A central polypurine tract/central termination sequence creates a "DNA flap" that increases nuclear importation of the viral genome during target-cell infection. The cPPT/CTS element improves vector integration and transduction efficiency. |
|
Kozak |
The Kozak consensus sequence plays a major role in the initiation of the translation process. |
|
T2A |
2A self-cleaving peptides, or 2A peptides, is a class of 18–22 aa-long peptides, which can induce ribosomal skipping during translation of a protein in a cell. |
|
SV40 early pA |
Simian virus 40 early polyadenylation signal. It further facilitates transcriptional termination after the 3' LTR during viral RNA transcription during packaging. This elevates the level of functional viral RNA in packaging cells, thus improving viral titer. |
Table 3. Other Commonly used Lentiviral Vector Elements
Lentiviral Vector Construction
After selecting the promoters, fluorescent tags and screening markers that meet the experimental requirements, the next step is to obtain the target fragments with restriction sites through direct synthesis or PCR amplification. The direct synthesis method is to directly synthesize according to the sequence of the target gene. The PCR amplification method is to extract the RNA of the sample with high expression of the target gene. Then, the cDNA obtained by reverse transcription is used as a template, and corresponding primers are designed to amplify the target fragment.
Subsequently, the target fragment can be ligated into the vector. Restriction enzyme cuts the target fragment with the restriction site and the vector backbone, and then connects the two by ligase to form a recombinant vector with the target fragment. Transform the amplified recombinant vector into competent cells and then confirm the correct construction of the recombinant vector by sequencing.
Lentiviral Packaging
Finally packaged into a lentivirus plasmid, the recombinant vector and 3 helper plasmids for virus packaging were co-transfected into 293T cells, the transfection efficiency was confirmed by observing the fluorescence, the supernatant where the lentivirus was collected by ultracentrifugation and filtered, then concentrate with PEG-NaCl, purify the lentivirus through a sucrose cushion, and finally perform titer determination and sterility testing.
Advantages of Lentivirus Vector Plasmids
Using the plasmid to overexpress foreign genes has the advantages of simplicity, quick turnaround, and low cost. Therefore, most laboratories prefer to use the plasmid method. But conventional plasmids cannot meet our needs if we want to overexpression experiments that have special requirements, such examples include difficult-to-transfect cells, which require stable genetic targeting and extremely high transfection efficiency.
The advantages of lentiviral vector when constructing overexpression vector
1. Stable integration and expression of foreign genes. The lentivirus integrates foreign genes into the host cell genome, so that they will be inherited stably as the host cells divide and pass through progeny.
2. Efficient transduction efficiency. The lentivirus with extremely high titer can be packaged, and the VSV-G protein of lentivirus has a wide affinity and can infect almost all mammalian cells. Therefore, the use of lentiviral vectors has extremely high infection efficiency and transduction efficiency.
3. Uniform gene copy number. Because the copy number of cells obtained by conventional/transient plasmid transfection is often uneven, some cells have high copy numbers, and some cells have low or no copy numbers. This results in differences in the expression of the target gene in different cells, making each There are differences in the genetic phenotypes of the cells. The number of gene copies in cells obtained by lentiviral transduction is relatively uniform.
Why Choose Cyagen
Cyagen can provide lentivirus (LV), adeno-associated virus (AAV), adenovirus (ADV) packaging services. We ensure the reliability of virus packaging quality from the aspects of virus titer, purity and activity, and may apply these alongside the construction of various cell models, in vivo injection of living animals, gene therapy research, and other fields. Our results have been published in many articles. References and reports in the literature help promote the smooth development of the experiment.
References:
[1] Srinivasakumar N. HIV-1 vector systems. Somat Cell Mol Genet. 2001 Nov;26(1-6):51-81. doi: 10.1023/a:1021074613196. PMID: 12465462.
[2] Maier P, von Kalle C, Laufs S. Retroviral vectors for gene therapy. Future Microbiol. 2010 Oct;5(10):1507-23. doi: 10.2217/fmb.10.100. PMID: 21073311.
[3] Al-Allaf FA, Coutelle C, Waddington SN, David AL, Harbottle R, Themis M. LDLR-Gene therapy for familial hypercholesterolaemia: problems, progress, and perspectives. Int Arch Med. 2010 Dec 13;3:36. doi: 10.1186/1755-7682-3-36. PMID: 21144047; PMCID: PMC3016243.
[4] Tolmachov, Oleg E., Tanya Tolmachova, and Faisal A. Al-Allaf. "Designing lentiviral gene vectors." Viral Gene Therapy. InTech (2011): 263-284.
We will respond to you in 1-2 business days.