
With the enhanced control of rat embryos and the use of Targeted Gene Editing technology, the scope of applications for rats as experimental model animals in basic research, drug screenings, and preclinical drug evaluations has expanded. However, in regards to the Cre-LoxP system – an important conditional gene expression construct - the application of rats has been seriously hindered due to the limited availability of Cre driver rat strains compared with mouse strains. The Cre-LoxP system has been widely used in mice, and many genes have corresponding LoxP mice, as well as abundant Cre mouse resources with specific promoters. However, due to the delayed development of gene-editing technology in rats, there are much lower numbers of LoxP or Cre rats connected to a tissue-specific promoter compared to those that are available for mice.
Cyagen continuously optimizes the production process to overcome multiple technical difficulties – such as the low fertilization rate of superovulated rat embryos, the high rate of abnormal embryos, the large differences between egg cell lines and individuals, and the thicker and more flexible nuclear and plasma membranes which make injections more difficult - to optimize our production efficiency with creating more complex conditional gene knockout rat models. In addition, Cyagen can provide a variety of gene-editing rat services, such as gene knockout (KO), point mutation, and gene knock-in (KI) across diverse background strains such as SD, Wistar, Long Evans, F344, and Brown Norway rats. Cyagen is committed to developing the best rat model for your research. Our efforts to support rat model research include the creating a repository of Cre models, including rat lines, to be available to researchers worldwide.
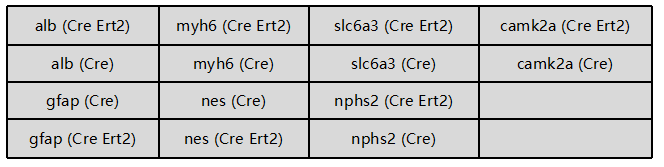
Note: Cyagen can provide pricing accommodations to researchers interested in Cre lines not currently available in our listings. Contact us to inquire about your conditional model needs and see how we can help develop a custom Cre strain to support your field of study.
Considering that Cre rats need to be crossed with LoxP rats for one to two generations to obtain conditional gene-editing animals alongside the fact that Cre enzymes have inefficiencies and probability of leakage – the breeding approach may affect the progress of scientific research. To address these potential drawbacks, virus-induced overexpression/knockout (KO) /knock-in (KI) /point mutation of rat models came into being.
Currently, there are three main viral vector expression systems in use, namely adenovirus, lentivirus, and adeno-associated virus. These viral systems have their own advantages and disadvantages:
Figure 1. Comparison of three common viral vectors
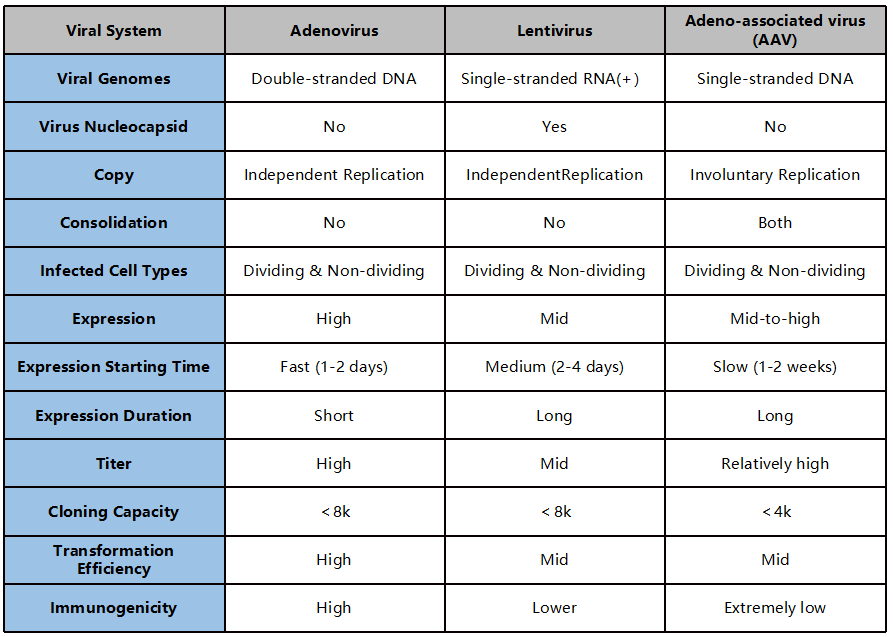
Based on the comparison of the above three viral vector expression systems for the generation of conditional gene expression animal models, it may be concluded that AAV vector-mediated tissue-specific expression has a better effect than the other two viral systems.
It should be noted that the tissue-specific expression produced by adeno-associated virus (AAV) injection is not just a time-saving solution. Due to the uncertainty of in vivo experiments in life science research, cross-validation with multiple methods can also help to improve the significance of your research results.
Figure 2. Tissue specificity of different Adeno-associated viruses (AAVs)
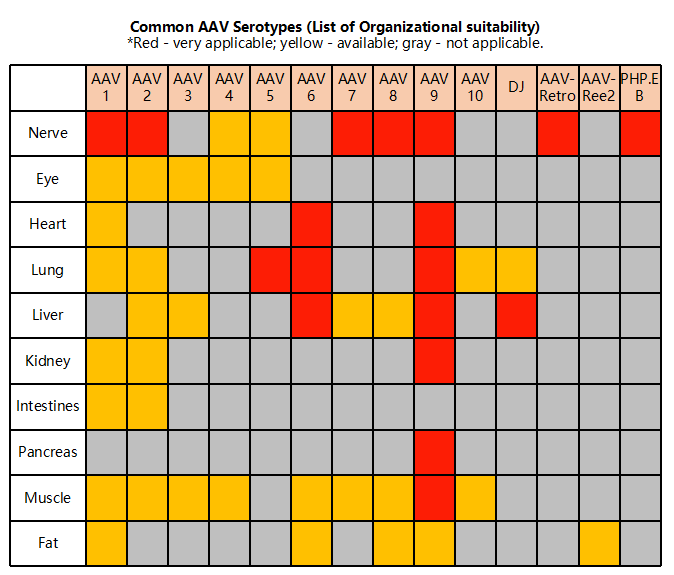
Additionally, adeno-associated viruses (AAVs) are often used in combination with Cre or LoxP rats. Although scientists continue to develop more LoxP and Cre rats, this process does not happen overnight – especially with the problems posed by Cre enzyme leakage in rats, which may lengthen the development time. The construction of viral vectors takes much less time than breeding animals to meet the volume required for experiments. Therefore, the most efficient strategy is to use AAV virus to perform tissue-specific operations on existing rats to obtain preliminary results, while developing Cre and LoxP rats. This approach can be used to study and verify the phenotype of animals quickly, saving time and allowing rapid evaluation of the research strategy and direction.
In recent years, Cre-LoxP animals have matured in the study of the spatial specificity of genes, and there are even cases where the transferred promoters are expressed in multiple tissues simultaneously. For tissue-specific Cre expression models, the expression of Cre in other areas would interfere with the accuracy of research results. This problem can be solved with fixed-point injection of AAV, which demonstrates a very strong regional specificity, into the target tissues.
To make better use of the gene-editing mice on hand, the AAV injection protocol can be divided into two types: 1) if you have LoxP rats already, you can inject with AAV packaging tissue-specific Cre; 2) conversely, if you have Cre rats, the virus can be injected with the target gene or other elements connected to the LoxP site. In general, tissue specific promoter for Cre recombinase with AAV Packaging is more commonly used.
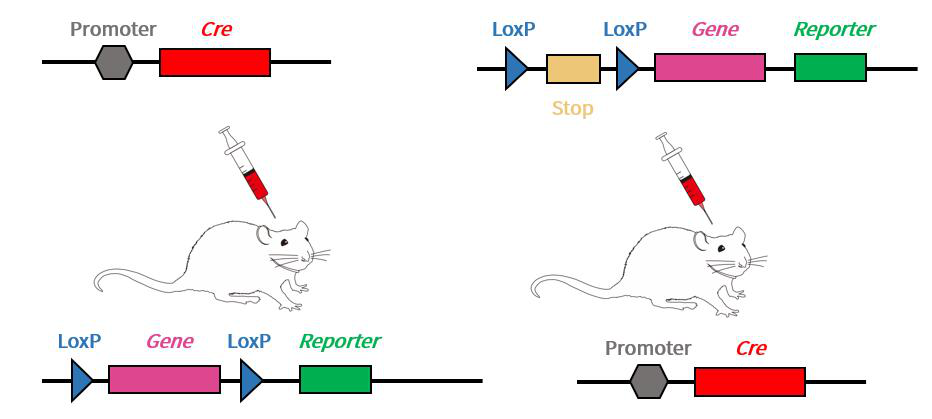
Figure 3. AAV viral packaging DNA combined with mice for conditional expression
Although AAV-mediated conditional gene editing has the many advantages mentioned above, its disadvantages should not be ignored:
Figure 4. Comparison of viruses and gene-edited mice for developing conditional knockout models
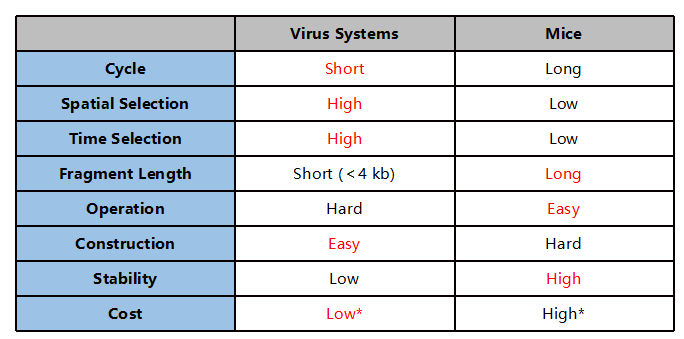
*Positional injection and short-term experiments have advantages.
Considering the pros and cons of the two methods for developing conditional animal models can help ensure their optimal use suited to your research plan. When conditions permit, AAV-assisted conditional knockout animals and traditional Cre-LoxP animals can be complementary and mutually validating in vivo models. With the advantages that rats provide for human disease research and recent advancements in gene editing among the species, rats are becoming an indispensable tool for biomedical research.
References:
We will respond to you in 1-2 business days.