
When it comes to eye care, people often overlook that eye diseases can also exist as a complication of other health conditions. When a diabetic person suffers from hyperglycemia or high blood pressure, it may result in damage to the blood vessels in the eye that may lead to diabetic retinopathy (DR). Undiagnosed diabetic retinopathy often leads to cases of preventable blindness, so early detection is key to effective treatment and prevention of blindness.
High Prevalence of DR Among Diabetic Patients
Diabetic retinopathy (DR) is a common microvascular complication in diabetic patients. In the United States, patients with DR account for 40% of the total number of those with type 2 diabetes and 86% of those with type 1 diabetes. A cross-sectional study based on the Chinese population showed that in rural China, there are as many as 21.1 million people with diabetes over the age of 30, of which about 9.2 million (roughly 44%) have DR. In addition, cohort studies from the United Kingdom, Australia, and other countries in Asia also reported a prevalence of DR ranging from 20% to 50% of the total diabetic population.
Clinical Classification of DR
According to the development stage and severity of DR, it is clinically divided into two subtypes: nonproliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). The first three of four stages are classified as mild, moderate, and severe NDPR. The fourth is PDR, an advanced stage of DR characterized by retinal pathological neovascularization and fibrovascular proliferation. Its further complications, vitreous hemorrhage (VH) and tractional retinal detachment (TRD), can cause severe visual impairment and even blindness in patients. In a 10-year cohort study of the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) in the United States, 17% of diagnosed DR patients developed severe vision-threatening PDR. Another 25-year cohort study showed that over time, 97% of patients with type 1 diabetes developed DR, of which 43% developed PDR.
Animal Models of DR and Related Diseases
Animal models are important research tools for understanding disease pathogenesis. Appropriate animal models are also of great benefit to the evaluation of the clinical efficacy of drugs.
Animal models of DR can be established by various modeling methods such as spontaneous hyperglycemia, surgery, or gene editing.
Genetic mouse models of DR mainly include Akita, non-obese diabetic (NOD), db/db, Kimba, and Akimba, which vary in inheritance pattern, disease etiology, pathology, and disease progression.
Figure Note: On the Rare Disease Data Center (RDDC), users can learn about relevant animal models by entering and searching for their disease of interest.
Drug-induced models have become commonly used animal models for DR due to their convenient modeling and strong simulation of pathological changes, mainly including diabetic models induced by streptozocin (STZ) and alloxan (AXL). STZ is the most commonly used drug for inducing diabetes, and its pharmacodynamic mechanism is mainly to cause hyperglycemia by destroying pancreatic β cells. In STZ-induced diabetic rats, BRB destruction occurs at 2 to 4 weeks of age; at 4 to 6 weeks of age, glial cell apoptosis is increased, accompanied by a reduction in retinal outer nuclear layer thickness and neuronal cells, followed by photoreceptor cell death. Thickening of the basement membrane and loss of pericytes are the basic pathological changes of DR, and the structural and functional integrity of pericytes and endothelial cells plays an important role in maintaining the stability of retinal capillaries. The STZ-induced diabetes animal model can reproduce the above pathological changes and is an ideal animal model for studying the early pathological changes of DR.
Studies have shown that STZ can: induce retinal hemorrhage, vascular disease, venous thrombosis and proliferative retinopathy in rabbits; induce ischemic retinopathies with cotton-wool spots and hyperfluorescence spots in monkeys; induce increased retinal BRB permeability, thinning of the inner nuclear layer (INL) and ganglion cell layer (GCL), and thickening of the capillary basement membrane in pigs. The STZ-induced DR animal model can better simulate the common clinical features in terms of pathological changes and serves as a widely used platform for the research on the pathogenesis and treatment of DR[3].
Diet-induced rodent models of eye diseases have been around for over 50 years. Mice on a high-fat, high-sugar diet develop hyperglycemia at 6 weeks, decreased retinal endothelial cells after 15 months, and develop lesions of microaneurysms and retinal thickening after 21 months. After 4 weeks of feeding the rats with a high-fat and high-sugar diet, the sensitivity of the rat's cone photoreceptors to light decreases. However, the disadvantage of this model is that it takes longer to develop since the onset of retinopathy is slower, but the advantage is that rodents live longer than other models.
Therefore, the most commonly used animal model of DR is to combine diet-induced with STZ injection. The principle of this model to simulate the pathogenesis of type 2 diabetes is to induce insulin resistance through a high-fat and high-sugar diet, and then inject low-dose STZ to damage islet function and cause hyperglycemia. Studies have shown that this approach can lead to retinal thinning and structural disorders, as well as increased expression of vascular endothelial growth factor. Therefore, diet-induced animal models are commonly used in long-term observation and drug screening experiments on DR, and are rarely used in routine experiments [4].
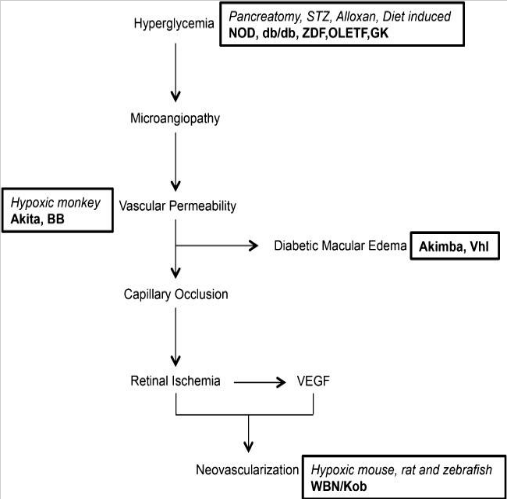
Pathogenesis and animal models of DR[5]
Diabetes mellitus (DM) can damage the retina and cause DR, making these pathologies closely related. DM is a syndrome composed of hyperglycemia with obvious heterogeneity and its acute and chronic complications, mainly caused by genetic, environmental, immune, and other factors. It has become the third major chronic disease threatening human health after cancer and cardiovascular disease. The American Diabetes Association (ADA) divides diabetes into type 1 diabetes (T1DM), type 2 diabetes (T2DM), and other special types of diabetes and gestational diabetes. Animal models of DM play an important role in the study of the disease and are generally divided into spontaneous, induced, and genetically engineered animal models of diabetes. Cyagen provides a variety of diabetic mouse models, such as type 2 diabetes mouse model (T2DM mice) and type 1 diabetes mouse model (T1DM mice), and phenotype analysis services.
Cyagen Accelerates Your Ocular Gene Therapy Development
There are a large number of patients with hereditary ophthalmic diseases worldwide. As a comprehensive contract research organization (CRO) solution provider, Cyagen has established a one-stop ocular gene therapy development platform to overcome the above obstacles.
With 16 years of experience in the field of custom animal models, Cyagen has independently developed a series of gene editing models targeting ophthalmic diseases, such as diabetic retinopathy (DR), retinitis pigmentosa (RP), retinal degeneration, Leber congenital amaurosis 2 (LCA2), Leber congenital amaurosis 10 (LCA10), age-related macular degeneration (ARMD), and corneal endothelial dystrophy.
Cyagen can provide you with genetically engineered animal models, fully-humanized mouse models, and surgical models to accelerate your preclinical pharmacodynamics evaluations. Our Preclinical Ophthalmology Research Solutions brings together a unique combination of standardized and professional resources, including The Rare Disease Data Center (RDDC), mature technical capabilities, and many successful cases to provide customized services suited to your ophthalmic research.
For further support, please do not hesitate to contact us or email service-apac@cyagen.com with your project details. Our experts are ready to help with your next research model!
[1] Araújo RS, Silva MS, Santos DF, et al. Dysregulation of trophic factors contributes to diabetic retinopathy in the Ins2 mouse [J]. Exp Eye Res, 2020, 194: 108027.
[2] Li CR, Sun SG. Rodent model of spontaneous diabetic retinopathy[J].Int Rev Ophthalmol, 2010, 34(2):119-122
[3] Lai AK, Lo AC. Animal models of diabetic retinopathy: summary and comparison [J].J Diabetes Res, 2013, 2013: 106594
[4] Clarkson-Townsend DA, Douglass AJ, Singh A, et al. Impacts of high fat diet on ocular outcomes in rodent models of visual disease[J]. Exp Eye Res, 2021, 204: 108440
[5] Olivares, A. M., Althoff, K., Chen, G. F., Wu, S., Morrisson, M. A., DeAngelis, M. M., & Haider, N. (2017). Animal Models of Diabetic Retinopathy. Current diabetes reports, 17(10), 93.
We will respond to you in 1-2 business days.