
In the previous article, we discussed preclinical studies of diabetic retinopathy (DR) and introduced some common animal models of DR. Over time, the vision of DR patients will gradually become blurred as the abnormal blood vessels associated with the condition can gradually pull the retina away from the back of the eye. Additional signs of retinal detachment include eye floaters (dark spots) in the field of vision and difficulty seeing faraway objects, as if the world in front of them is covered with a layer of fog.
Diabetic retinopathy (DR) usually causes progressive damage to patients, so it is particularly important to take appropriate treatment measures according to the degree of disease progression. Today, we take a look at two important treatments for DR: surgical therapy and gene therapy.
At present, the treatment of DR mainly relies on surgery. Through the rapid development of medical technology in recent years, the therapeutic effect of laser photocoagulation has also been continuously improved.
Pan retinal photocoagulation (PRP), proposed in the 1960s, can prevent further vision loss in proliferative DR patients. The main mechanism of action of PRP is that photocoagulation-induced scars relieve the hemodynamics of retinal microcirculation and improve retinal oxygenation, thereby inhibiting the production of vascular endothelial growth factors and other factors to achieve therapeutic purposes. However, due to the destructiveness of the laser, PRP has certain side effects, such as inducing visual field scotoma, and may have complications such as vitreous hemorrhage and secondary neovascularization.
When patients with proliferative DR develop vision-threatening complications, such as vitreous hemorrhage or tractional retinal detachment, the main treatment method is pars plana vitrectomy (PPV), supplemented by PRP.
Removal of the vitreous body, on the one hand, clears the blood in the eye so that the fundus of the patient can be observed; on the other hand, it reduces the concentration of vascular endothelial growth factor (VEGF) and inflammatory factors, which further improves the intraocular microenvironment. Intraoperative peeling of fibrovascular membranes (FVM) can relieve the traction effect and restore the anatomical structure of the retina. Therefore, PPV therapy is of great significance in clinical proliferative DR patients.
However, due to the fragility of the neovascular area, intraocular hemorrhage is often unavoidable (especially when removing FVMs), which greatly increases the difficulty of PPV surgery. In previous clinical cases of fundus diseases, doctors mainly suppressed active bleeding during surgery by increasing intraocular pressure or electrocoagulation. However, prolonged high intraocular pressure can lead to corneal epithelial edema and reduce the clarity of the surgical field. And excessive coagulation can lead to retinal necrosis and aggravate postoperative inflammatory responses. The use of a vitreous cutter to clear multiple retinal hemorrhages or to separate FVMs can cause new retinal breaks. Therefore, PPV therapy in patients with proliferative DR has a certain failure rate due to the difficulty of the procedure.
Compared with traditional medical methods, gene therapy is an innovative research hotspot that is developing in full swing around the world. Similarly, gene therapies also bring new opportunities for the diagnosis and treatment of fundus diseases such as DR. Herein, we share some case studies of gene therapy targeting retinal diseases.
Colella et al[1] constructed a VEGF transgenic mouse model and used an AAV vector to mediate the gene transfer of the soluble receptor sFlt-1. By injecting the vector into the subretinal space, the therapeutic gene is expressed in the eye, thereby effectively inhibiting the occurrence of microvascular abnormalities in the retina.
Upregulation of VEGF is associated with neovascularization in the eye. Ambati et al[2] constructed a mouse model for retinopathy of prematurity (ROP) and used adenovirus (ADV) and AAV vector to mediate sFlt-1 gene transfer, respectively. The results showed that normal mouse eyes had low expression of VEGF and high expression of sFlt-1, and sFlt-1 inhibited the expression of VEGF to protect the eyes. However, VEGF expression was significantly increased in the eyes of ROP mice, while sFlt-1 was inhibited. In the experiment, the high expression of the sFlt-1 gene in cells mediated by the virus has a therapeutic effect on ROP mice.
Díaz-Lezama et al[3] used an inhibitor of blood-retinal barrier damage mediated by an AAV vector to explore its efficacy on diabetic macular edema (DME). First, streptozotocin (STZ) was used to induce diabetes in rats; after 2 weeks, the hemorrhagic-retinal barrier was damaged by detecting the albumin content in the vitreous body, and the albumin content was significantly increased. However, the albumin content in the eyes of rats injected with AAV overexpressing VEGF antibody remained normal two weeks after the induction of diabetes, which proved that the gene therapy can protect the blood-retinal barrier.
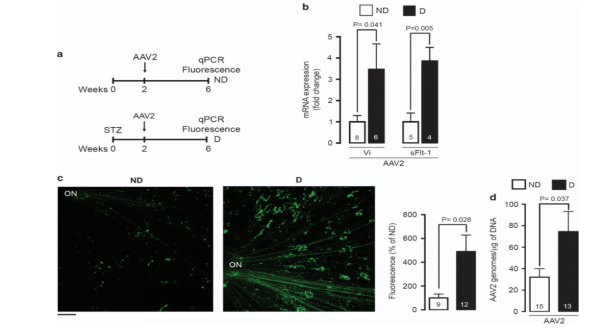
Figure 1. Stretched preparation of retina and qPCR prove that AAV2 can successfully transduce target genes[3].
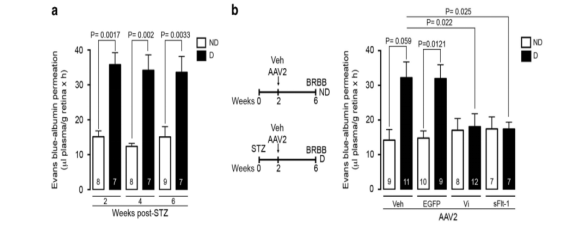
Figure 2. The albumin content of diabetic rats before and after injection of AAV overexpressing VEGF antibody[3].
As a comprehensive contract research organization (CRO) solution provider, Cyagen recognizes ophthalmic diseases as a breakthrough point for gene therapy and has established an ophthalmic gene therapy platform. We can provide genetically engineered animal models, fully-humanized mouse models, surgical models, and downstream services to accelerate your research through preclinical pharmacodynamics evaluations. If you have questions or want help with your ophthalmology drug development program, you’re invited to have a complimentary talk with our experts!
|
Diseases |
Genes |
Gene Variants |
|
Leber Congenital Amaurosis type 2(LCA2) |
RPE65 |
KO, MU (p.R44*) |
|
Leber Congenital Amaurosis type 10(LCA10) |
CEP290 |
Humanization |
|
Leber Congenital Amaurosis (LCA) |
RDH12 |
KO |
|
Retinitis pigmentosa (RP)
|
MERTK |
KO+CKO |
|
PDE6B |
KO |
|
|
RHO |
KO+CKO, humanization, humanization (MU: P23H) |
|
|
RPGR |
KO |
|
|
CRB1 |
KO |
|
|
RD1 |
Mut |
|
|
RD10 |
Mut |
|
|
Age-related macular degeneration (AMD) |
hVEGF |
KI, TG |
|
Stargardt disease |
ABCR/ABCA4 |
KO, humanization |
|
Choroideremia |
CHM |
CKO |
|
Usher syndrome type 2 |
USH2A |
Humanization |
|
Corneal Endothelial Dystrophy |
TCF4 |
KO+CKO |
|
Autosomal Recessive Bestrophinopathy |
BEST1 |
KO |
|
Retinal degeneration
|
Prph2/Rds |
KO |
|
Tub |
KO |
|
|
Achromatopsia |
CNGA3 |
KO+CKO |
Table 1. Cyagen-developed ophthalmic disease mouse models for gene therapy research.
[1] Colella, P., & Auricchio, A. (2010). AAV-mediated gene supply for treatment of degenerative and neovascular retinal diseases. Current gene therapy, 10(5), 371–380.
[2] Ambati, B. K., Patterson, E., Jani, P., Jenkins, C., Higgins, E., Singh, N., Suthar, T., Vira, N., Smith, K., & Caldwell, R. (2007). Soluble vascular endothelial growth factor receptor-1 contributes to the corneal antiangiogenic barrier. The British journal of ophthalmology, 91(4), 505–508.
[3] Díaz-Lezama, N., Wu, Z., Adán-Castro, E., Arnold, E., Vázquez-Membrillo, M., Arredondo-Zamarripa, D., Ledesma-Colunga, M. G., Moreno-Carranza, B., Martinez de la Escalera, G., Colosi, P., & Clapp, C. (2016). Diabetes enhances the efficacy of AAV2 vectors in the retina: therapeutic effect of AAV2 encoding vasoinhibin and soluble VEGF receptor 1. Laboratory investigation; a journal of technical methods and pathology, 96(3), 283–295.
[4] Methods & Clinical Development, 24, 210-221.
[5] Buck, T. M., & Wijnholds, J. (2020). Recombinant adeno-associated viral vectors (rAAV)-vector elements in ocular gene therapy clinical trials and transgene expression and bioactivity assays. International journal of molecular sciences, 21(12), 4197.
[6] Telias, M., Denlinger, B., Helft, Z., Thornton, C., Beckwith-Cohen, B., & Kramer, R. H. (2019). Retinoic acid induces hyperactivity, and blocking its receptor unmasks light responses and augments vision in retinal degeneration. Neuron, 102(3), 574-586.
[7] Lee, S. H., Kim, Y. S., Nah, S. K., Kim, H. J., Park, H. Y., Yang, J. Y., ... & Park, T. K. (2018). Transduction patterns of adeno-associated viral vectors in a laser-induced choroidal neovascularization mouse model. Molecular Therapy-Methods & Clinical Development, 9, 90-98.
We will respond to you in 1-2 business days.