
Alzheimer's disease (AD) is the most common age-related neurodegenerative disorder, which affects 20 to 30 million individuals worldwide now. Specific mutations in TREM2 (Triggering Receptor Expressed on Myeloid Cells 2) have been confirmed to increase the risk of developing late-onset AD. Here, we review the current data detailing the function of TREM2 and its role in Alzheimer's disease (AD).
TREM2 Aliases: Triggering Receptor Expressed on Myeloid Cells 2, TREM-2, PLOSL2, Triggering Receptor Expressed on Monocytes 2
Related Diseases: Alzheimer's disease (AD), Nasu-Hakola disease (NHD), Frontotemporal Dementia (FTD)
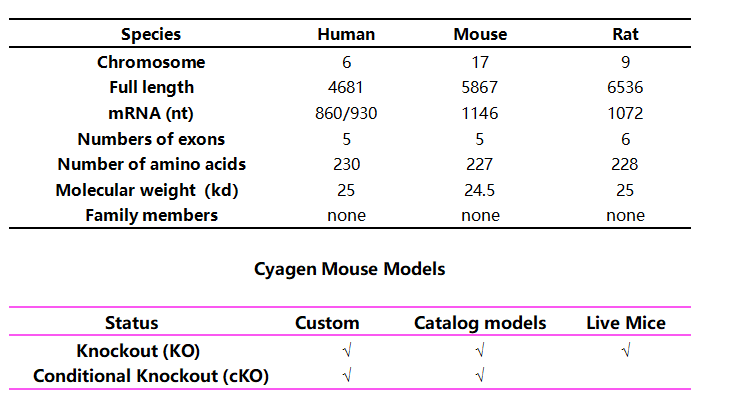
Note: The mark ‘√’represents the research-ready models that are available from our Cyagen AI Knockout Mouse Model eBank.
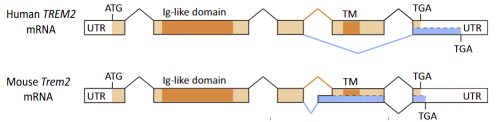
Figure 1. Human and mouse TREM2 gene structure. Human TREM2 has two protein expression forms that with different functions, the longer one contains the intracellular domain, the shorter one deletes the fourth exon - without the intracellular domain.
The TREM2 (Triggering Receptor Expressed on Myeloid Cells 2) gene encodes a transmembrane receptor protein of the immunoglobulin superfamily. TREM2 is produced by microglia in the brain and mainly regulates the survival and activation of microglia. This gene is related to diseases such as Nasu-Hakola disease (NHD), Alzheimer's disease (AD), and Frontotemporal dementia (FTD). Mutations in TREM2 can increase the incidence of Alzheimer's disease to three times the normal level.
Currently, there are 67 mutations of TREM2 that have been identified, of which, 40 are related to Alzheimer's disease (AD), which includes only one clear cause and 8 risk factors. Additionally, 11 mutations are known causes of Nasu-Hakola disease (NHD). Lastly, 8 of the identified mutants are causative factors in Frontotemporal Dementia (FTD), with 3 being risk factors. It is notable that Q33X is also common pathogenic mutation for the aforementioned diseases linked to TREM2 gene variants.
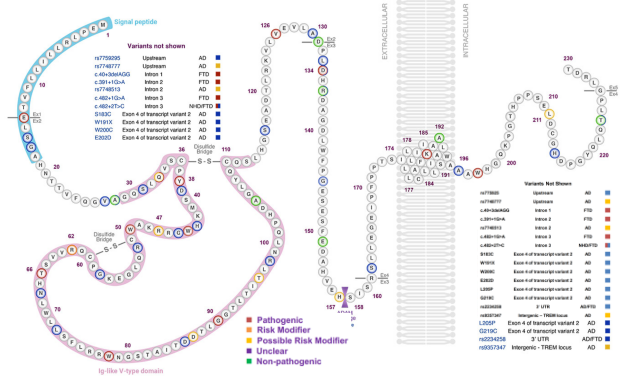
Figure 2. TREM2 protein structure and mutation sites.[5]
A wide range of anionic molecules serve as ligands of TREM2, including bacterial products, DNA, and phospholipids. The main ligands of TREM2 are apolipoproteins (APOE), lipoproteins (e.g. HDL, LDL), and β-amyloid (Aβ). Additionally, given that TREM2 and APOE both present risk variants linked to late-onset AD, their ligand interactions are of particular interest.
Upon TREM2 ligand binding, the immunoreceptor tyrosine-based activation motif (ITAM) in the intracellular domain of adaptor proteins becomes phosphorylated, leading to the activation of downstream signaling molecules. These adaptor proteins - DNAX-activating protein of 12 kDa (DAP12) and DAP10 – are critical for recruiting the intracellular signal transduction machinery required for downstream signaling.
The TREM2/DAP12-mediated signaling pathway regulates microglial activity – including cell proliferation, survival, phagocytosis, and inflammation – as well as other signaling pathways. Given the varying roles of activated microglia across disease models, the effects of TREM2/DAP12 signaling may differ among disease models.
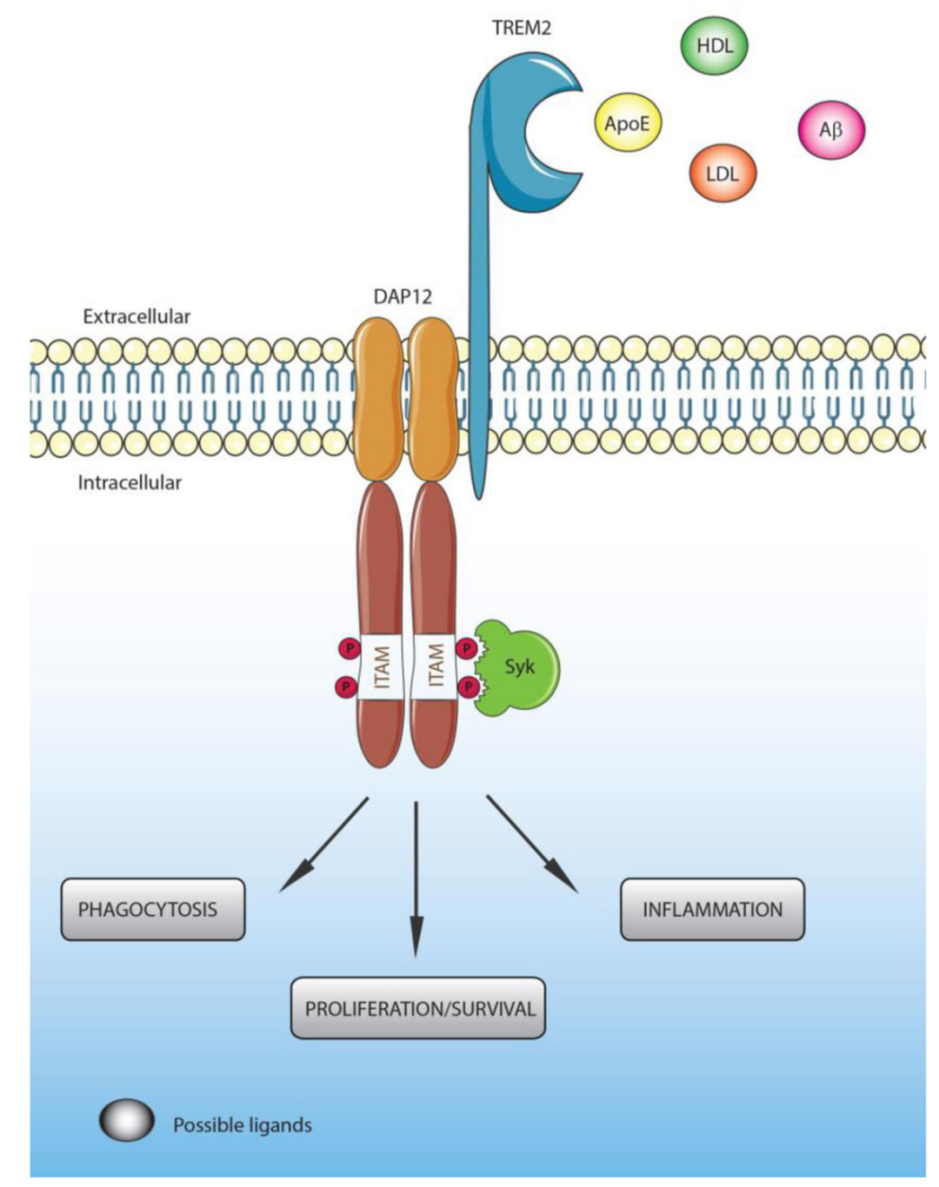
Figure 3. TREM2/DAP12 signaling [1].
Full-length TREM2 undergoes cleavage by ADAM (a disintegrin and metalloproteinase domain containing protein) family proteases, which produces soluble TREM2 (sTREM2). The sTREM2 enters the intercellular space to activate microglia, thereby enhancing the viability of the cells. After being cut, the normal function of TREM2 also inhibits the production of Aβ.
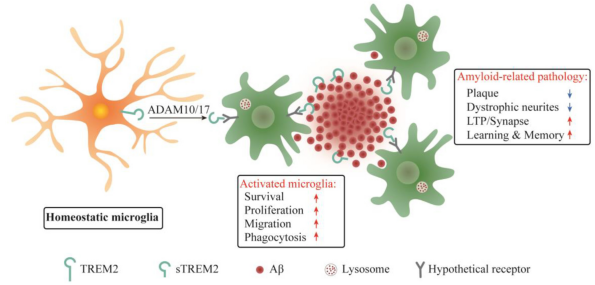
Figure 4. Function of soluble TREM2 (sTREM2) [2]
We will respond to you in 1-2 business days.