
CAR T cell therapy research has developed rapidly in recent years, largely based on the remarkable efficacy of CAR T therapy in hematological malignancies, and scientists are moving quickly to apply this innovative immunotherapy to the treatment of solid tumors, including sarcomas, carcinomas, and lymphomas. Herein, we review the background of CAR T cell therapy in solid tumor treatment, common research targets, CAR T optimization strategy, and clinical research progress.
Chimeric antigen receptor T (CAR T) cell therapy refers to genetically modified T Cells that can recognize specific antigens on the surface of tumor cells and kill tumor cells by releasing a variety of immune factors to achieve the purpose of tumor treatment. Developing CAR T cell therapy involves the transfer of genetic material with the tumor-specific antigen recognition domain and T cell activation signal into T cells through gene modification technology.
An increasing number of clinical trials of CAR T therapy targeting solid tumor antigen targets have been carried out in recent years. According to the data provided by ClinicalTrials.gov, there were 1,800 cell-based clinical trials in progress as of April 2022, an increase of 33% compared to last year. Among these cell-based clinical trials, those targeting solid tumors accounted for 43%, an increase of 44% compared to last year, which actually exceeded the increase in hematological malignancies (which only increased 25% from 2021). In the last year, CAR T therapies for solid tumors developed more rapidly than even for hematological malignancies - demonstrating the growing potential of the CAR T cell therapy approach.
In recent years, CAR T studies targeting various solid tumor antigens have gradually increased and a considerable number have entered the early stage of clinical trials.
The killing activity of CAR T cells against tumor cells mainly depends on the recognition of tumor-associated antigens (TAAs), which are often highly expressed on the surface of tumor cells, but also expressed to a certain extent on other normal tissue cells. Therefore, while removing tumor cells, CAR T cells may also mistakenly attack normal tissue cells. These kinds of off-target effects may result in serious side effects from killing non-tumor cells, making a non-specific CAR T therapy unable to achieve the desired therapeutic effect.
Compared with hematological malignancies, it is more difficult for CAR T cells to penetrate solid tumor tissue through the blood system. On the one hand, solid tumors can perform immune evasion by interfering with T cell trafficking; on the other hand, due to chemokine-chemokine receptor mismatch, downregulation of adhesion molecules, and abnormal tumor neovasculature, it is difficult for CAR T cells to precisely navigate to the tumor. In addition, another characteristic of solid tumors is the presence of large numbers of tumor-associated fibroblasts in stromal cells. By secreting collagen, these cells form a dense fibrotic matrix that acts as a physical barrier to trap and prevent CAR T cell infiltration and attenuate their killing activity.
The tumor microenvironment (TME) is a microenvironment redesigned by cancer cells to promote their growth, which is characterized by low oxygen, low nutrition, low pH, and high permeability. This environment can promote tumor proliferation but is very unfavorable for the survival of CAR T cells. In addition, the TME also contains a variety of immunosuppressive cells such as tumor-associated macrophages (TAM), regulatory T cells (Tregs), myeloid-derived suppressor cells, MDSC), etc.; these immunosuppressive cells inhibit CAR T cells through multiple pathways, significantly reducing their killing efficacy.
To address these challenges, researchers have already performed a significant amount of preparatory and forward-looking work. The main coping strategies proposed thus far include:
In order to allow CAR T cells to simultaneously express pro-inflammatory cytokines that can change the characteristics of the tumor microenvironment and to prevent the continuous expression of these factors in the body from causing toxicity, some studies have designed CAR T cells to drive cytokine production after binding to the target expression, thereby limiting the action of cytokines to the vicinity of the tumor. Other strategies, such as expressing inhibitory CAR molecules, activating CAR molecules with dual targets, and increasing the affinity of scFv to change the specificity of CAR T cells, optimize their anti-tumor activity and safety.
Using CRISPR-mediated gene knockout technology can reduce the sensitivity of CAR T cells to the tumor microenvironment. For example, Tmunity Therapeutics uses gene editing technology to knock out the expression of endogenous T cell receptors (TCR) and PD-1 receptors in T cells, then express TCR targeting the New York esophageal squamous cell carcinoma 1 (NY-ESO-1) antigen - enabling CAR T cells to reach the solid tumor site more efficiently, thereby enhancing the tumoricidal effect of CAR T cells in the tumor microenvironment.
BioNTech has used liposomes to deliver the vaccine encoding CLDN6 mRNA. After injection into the body, antigen-presenting cells (APCs) in the spleen, lymph nodes, etc. can present the CLDN6 antigen to the surface. These APCs can promote the proliferation of CLDN6 CAR T cells. In addition, the combination of CAR T therapy with the immune checkpoint inhibitor PD-1 has also improved the therapeutic effect of CAR T therapy on solid tumors.
The top medical journal Nature Medicine published the latest study from the team of Carl June, the father of CAR T, which presents a new CAR T therapy that has also rekindled hope for their application in treatment of solid tumors. In the microenvironment of solid tumor metastatic castration-resistant prostate cancer (mCRPC), there are a variety of high-level inhibitory factors such as transforming growth factor-β (TGF-β), which can significantly reduce the efficacy of CAR T cells. The research team uses gene editing to target PSMA to inactivate the relevant receptors of CAR T cells, thereby resisting the inhibitory effect of TGF-β by blocking TGF-β signal transduction, and enhancing the antitumor ability of CAR T cells.
On the basis of the good curative effect obtained in the previous animal experiments, the therapy has undergone a phase I clinical trial. The results showed that in 13 treated mCRPC patients, CAR T cells expanded and persisted in vivo, and were significantly enriched in tumor tissue. In terms of efficacy assessment, 4 out of 13 patients had a decrease in Prostate-specific antigen (PSA) of ≥30% (Fig. 1-a); in addition, CT assessment showed that 5 patients (38.5%) maintained stable disease at 3-month radiological evaluation, and evidence of tumor regression was observed in one of the patients after cell therapy—its PSA decreased by 36% after cell infusion (Figure 1-b).
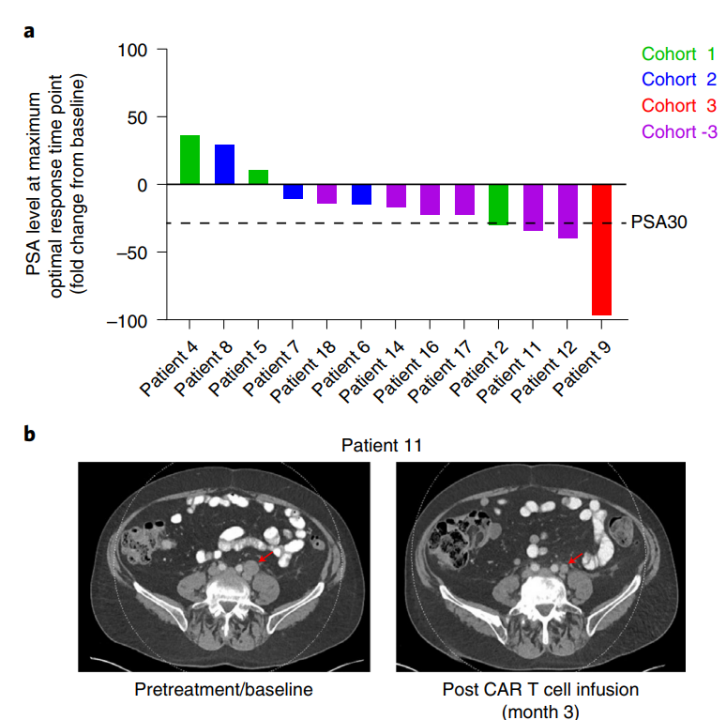
Figure 1. Pathological and radiological evaluation after CART-PSMA-TGFβRDN cell therapy[1]
This study is also the first clinical study of CAR T in the treatment of solid tumors, and the results confirm the feasibility and safety of anti-TGF-β CAR T cell therapy targeting PSMA for the treatment of solid tumors such as mCRPC, which has provided great motivation and confidence for CAR T therapy in tackling solid tumors.
There are still many difficulties in the effective treatment of solid tumors with CAR T therapy, but the efforts and exploration of scientists have never stopped. An increasing number of studies are aimed at circumventing the challenges of the tumor microenvironment and the factors that affect the anti-tumor effect of CAR T. By coupling these strategies with the potential of a combination therapy approach, we believe that we will definitely see more possibilities for CAR T therapy to conquer the clinical treatment of solid tumors.
Cyagen provides one-stop cell therapy research solutions supporting CAR T and other cell therapy development programs, including CAR virus preparation, the construction of tumor immune cells and animal models, and the entire process of in vivo/in vitro drug efficacy evaluation — accelerating the development of CAR T and cell therapy research.
[1] Narayan V, Barber-Rotenberg JS, Jung IY, et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial [published online ahead of print, 2022 Mar 21]. Nat Med. 2022;10.1038/s41591-022-01726-1.
We will respond to you in 1-2 business days.