
Leber Hereditary Optic Neuropathy (LHON) is a type of hereditary vision loss that has a sudden onset. It starts with a painless clouding or blurring in one or both eyes, and then worsens to decreased visual sharpness and loss of color vision. As we all know, the study of hereditary diseases often starts with knowing which chromosome the causative gene is located on. However, LHON is very special because its causative gene is not located on any human chromosome! Today, let’s unravel the mystery of this inherited rare disease.
Leber Hereditary Optic Neuropathy (LHON) is a maternally inherited optic neurodegenerative disease caused by mitochondrial DNA mutation that mainly affects the fundus. LHON was first described by the German ophthalmologist Theodor Leber (1840–1917) in 1871. LHON is rare and has the characteristics of incomplete penetrance, which means that carriers of the mitochondrial gene mutation may not develop the disease. The ratio of male to female patients with LHON is approximately 5:1, with a clear tendency to be more common in males, and the age of onset is usually 15 to 35 years old [1].
In addition to chromosomes, there is an important organelle in the cell that also carries part of the genetic information - the mitochondria. The DNA mutation that causes LHON is located in mitochondrial DNA (mtDNA). During fertilization, the sperm releases only its nuclear DNA into the egg cell, leaving the rest out. Therefore, all diseases caused by mtDNA mutations have the characteristics of maternal inheritance, as the mtDNA is inherited from the ovum.
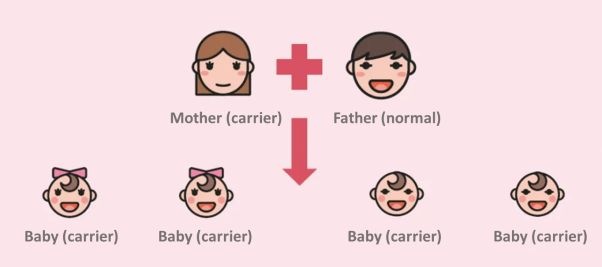
Figure 1. Maternal inheritance
The common mitochondrial DNA (mtDNA) primary mutation sites of LHON are 11778G>A, 3460G>A, and 14484T>C, accounting for more than 90% of all LHON mutations. These mutations can affect the function of mitochondrial respiratory chain complex I, resulting in decreased mitochondrial ATP synthesis and increased production of reactive oxygen species (ROS), resulting in ganglion cell apoptosis. In addition to the above-mentioned primary sites, more than 50 secondary mutation sites on mtDNA have been found so far. The penetrance of different genes, mutations, and the severity of the disease vary. Furthermore, nuclear gene mutations, environmental factors, and mtDNA haplotypes may all contribute to onset of disease pathogenesis.
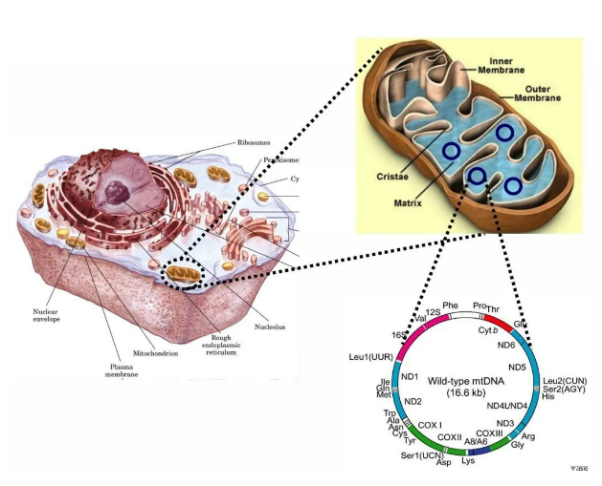
Figure 2. Mitochondria and mtDNA
The prevalence of LHON was 1/27,000 in North East England and 1/45,000 in a European population-based study. In Asia, the incidence rate is about 1.18/10000, and the m.11778G>A mutation accounts for about 87%-92.9% of the total APAC mutations, while it accounts for around 90% of total mutations in China. In other words, there are about 150,000 LHON patients with m.11778G>A mutation in China, based on total populations [2].
The main symptom of LHON is painless reduction in visual acuity that occurs successively in both eyes, usually over a period of weeks to months. In the early stage of the disease, fundus examination shows optic disc edema, telangiectasia, and looped/coiled peripapillary retinal vessel (microangiopathy). In the later stage of the disease, optic disc edema and telangiectasia subside, and eventually, the temporal side or all of the optic disc shows atrophic changes. Most of the symptoms of LHON patients are only manifested in the eye, but a few patients also have other systems affected, showing symptoms such as intellectual disability, epilepsy, hearing impairment, and dystonia.
European and North American expert groups have - by evaluating optical coherence tomography (OCT) images at various time points, medical history, best-corrected visual acuity, visual field, and other visual function indicators - redefined the clinical stages of LHON: asymptomatic, subacute, dynamic and chronic phases.
Ocular examination of asymptomatic patients may show no abnormalities, but may also have fundus changes with optic disc hyperemia and telangiectasia, as well as inferior and temporal nerve fiber layer thickening observed on OCT. Asymptomatic patients are carriers of the mutation, but have yet to exhibit symptoms of LHON.
The new clinical stages subdivide cases with a duration of less than one year into subacute and dynamic phases, which can better describe the changes of LHON patients at different stages [3]; patients with onset for less than 6 months are in the subacute phase. During this period, the patient's visual acuity declines rapidly and stabilizes by 4 to 6 months. A central scotoma is seen on visual field examination and progressively enlarges.
The duration of 6 months to 1 year after onset is the dynamic phase. There may be no significant changes in visual acuity during this period, but visual field examination and OCT images show that the damage is still progressing, and usually stops about 1 year after symptoms appear, reaching a plateau.
Patients enter the chronic phase 1 year after the onset of clinical symptoms.
In addition to the above-mentioned typical clinical stages, there are also subtypes of the disease such as slowly progressive, childhood-onset, and late-onset [4].
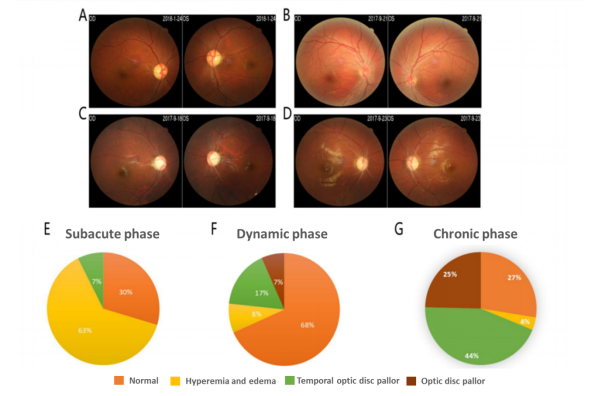
Figure 3. Analysis of fundus of LHON patients: (A) Fundus photography (normal optic disc); (B) Fundus photography (optic disc hyperemia and edema); (C) Fundus photography (temporal optic disc pallor); (D) Fundus photography (optic disc pallor); (E) Proportion of optic disc manifestations in patients with subacute phase LHON; (F) Proportion of optic disc manifestations in patients with dynamic phase LHON; (G) Proportion of optic disc manifestations in patients with chronic phase LHON [3].
There was no obvious change in Visual Evoked Potential (VEP) in the early stage of LHON patients, but latency delay and amplitude decrease may occur in the later stages.
Fundus Fluorescein Angiography (FFA) plays an important role in the diagnosis of LHON. In the acute phase, optic disc hyperemia, peripheral telangiectasia and tortuosity, and swelling of the nerve fiber layer appeared, but the FFA showed no fluorescein leakage. In the chronic phase, optic disc discoloration or pallor can be observed by FFA, while optic atrophy occurs in the advanced phase [5].
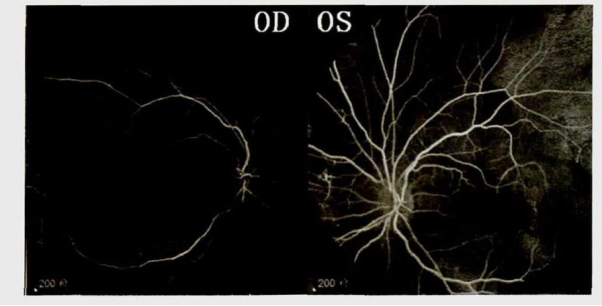
Figure 4. FFA of LHON in the acute phase. No fluorescein leakage was seen in or around the optic disc [4].
Optical Coherence Tomography (OCT) images can show the thickness of the retinal nerve fiber layer (RNFL) in patients with LHON in various stages. Some studies have shown that the RNFL in asymptomatic LHON patients is thinner than that in the control group. In the early stages of the disease, the RNFL of LHON patients thickens, and it becomes significantly thinner in the advanced stages. Temporal nerve fibers are involved first and most severely, some nasal fibers appear to be unaffected even in advanced disease stages, and diffuse damage to optic nerve fibers is more pronounced in males than in females. In patients with advanced vision recovery, some RNFL thickness may be preserved.
Visual field (VF) examination in patients with LHON usually shows central scotoma or paracentral scotoma. As the disease progresses, the visual field defect expands to the periphery [6].
The Magnetic Resonance Imaging (MRI) appearance of the optic nerve in patients with LHON is inconclusive. Occasionally, T2 hyperintensity in the optic tract and lateral geniculate body and enhancement of the optic nerve and optic chiasm can be detected. For those with other neurological manifestations, cranial imaging examination is required. Some patients have white matter lesions similar to multiple sclerosis (MS), and the pathology can also manifest as nonspecific white matter changes [7].
The Rare Diseases Data Center (RDDC) was developed by the Research Institute of Tsinghua, Pearl River Delta (RITPRD) with assistance from Cyagen Biosciences.
The RDDC database has collected and sorted out all information about rare diseases from international public biological resources, including related genes, mutations, phenotypes, and animal models of rare diseases, aiming to present them with added visualization capabilities. At present, the RDDC has collected information on over 20,000 genes and 14,000 human diseases - all freely accessible!
Users are not only able to better understand the information of corresponding rare diseases, but also can take advantage of various AI tools to predict pathogenicity and RNA splicing patterns. The RDDC tools are available to help speed up the research and development of treatments for rare diseases, try them out now:
Click the photo above for more information!
*Statement: RDDC data and tools are only for scientific research reference, and should not be used as the final conclusion in medical diagnosis and evaluation.
[1] Yeqing Doing, Qiping Wei, Ziyang Wang, Xuanchen Guo, Zitong Wang, Yanhong Sun. Clinical Experience of Wei Qiping in the Treatment of Leber Hereditary Optic Neuropathy from Liver [J]. Chinese Journal of Ophthalmology of Traditional Chinese Medicine, 2022, 32(05):367-371. DOI: 10.13444/j.cnki.zgzyykzz. 2022.05.007.
[2] Jiajia Yuan. Leber's Hereditary Optic Neuropathy Gene Therapy Studies [D]. Huazhong University of Science and Technology, 2020. DOI: 10.27157/d.cnki.ghzku. 2020.000280.
[3] Dan Wang.Clinical Characteristics, Retinal Nerve Fiber Layer Thickness and Cribriform Lamina Morphology of G11778A Leber Hereditary Optic Neuropathy [D]. Huazhong University of Science and Technology,2021. DOI: 10.27157/d.cnki.ghzku. 2021.000190.
[4] https://www.nrdrs.org.cn/app/rare/disease-list-article.html?index=62.
[5] Yani Liu, Jinjin Zhang, Xunlun Sheng, Shuang Zhang, Wenzhou Liu, Zhiqing Zhao, Weigang Xu. Clinical and Genetic Research of Leber Hereditary Optic Neuropathy [J]. Ningxia Medical Journal, 2020,42(09):777-779. DOI: 10.13621/j.1001-5949. 2020.09.0777.
[6] Jiawei Wang, Juan Zhao.Expert Consensus on the Diagnosis and Treatment of Leber's Hereditary Optic Neuropathy [J]. Ophthalmology, 2019, 28(05):328-335. DOI:10.13281/j.cnki.issn. 1004-4469. 2019.05.003.
[7] Xiaojun Zhang, Wenbin Wei. Walsh and Hoyt.Refined Clinical Neuro-Ophthalmology. Beijing: Science Press, 2009: 230-244.
[2] Hongmi Zou,Xiyuan Zhou.A Review of Studies on Retinoblastoma Disease Models[J]. Scientific Consulting (Technology · Management),2018(5):48-50.
[3] Leal-Leal C,Flores-Rojo M,Medina-Sanson A,et al.A multicentre report from the Mexican Retinoblastoma Group[J].Br J Ophthalmol,2004,88(8):1074-1077.
[4] Broaddus E,Topham A,Singh A D. Incidence of reti- noblastoma in the USA: 1975-2004 [J]. Br J Ophthal- mol,2009,93(1):21-23.
[5] Shields C L,Shields J A.Diagnosis and management of retinoblastoma[J].Cancer control,2004,15(11):317-327.
[6] Soliman SE, Racher H,Zhang C, et al. Genetics and molecular diagnostics in retinoblastoma-an update[J]. Asia Pac J Ophthalmol,2017,6(2):197-207.
[7] Bai S, Ren R,Li B, et al. Delay in the diagnosis of retinoblastoma in China[J]. Acta Ophthalmol, 2011,89(1):e72-e74.
[8] Corson TW, Samuels BC, Wenzel AA, et al. Multimodality imaging methods for assessing retinoblastoma orthotopic xenograft growth and development[J]. Plos One, 2014, 9(6):e99036.
We will respond to you in 1-2 business days.