
Acute pancreatitis (AP) is a common disorder that presents complex etiology, pathogenesis, and clinical manifestations - ranging from mild disease to sepsis and multisystem organ failure (MOF). Severe acute pancreatitis (SAP) causes damage to multiple organs that often results in necrosis – which can lead to 50%~60% fatality rates. At present, there is no specific treatment to prevent pancreatic necrosis. Since 1856, the establishment of the first acute pancreatitis (AP) animal model, which had played an important role in acute pancreatitis research. The main methods of pancreatitis animal model generation are as follows:
Methods: Retrograde injection of sodium taurocholate into the bile duct resulted in acute pancreatic hyperemia and hematoma in mice.
Detection Index: Acute hyperemia and edema of pancreas and saponification spots in abdominal cavity were observed.
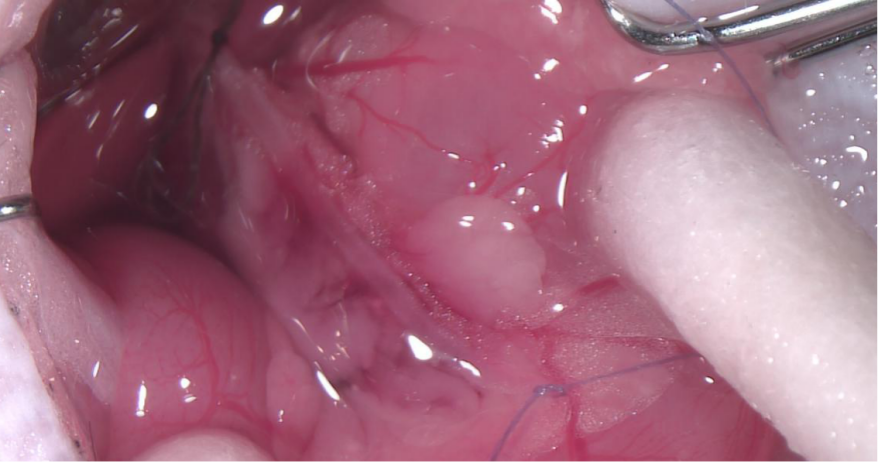
Picture 1: Acute hyperemia and edema of pancreas in 5 min after injection

Picture 2: Saponification spots were observed in abdominal cavity within 24-48 hours after injection
Acute Pancreatitis Modeling Course
Cyagen Professional Surgical Disease Models Team Will Elaborately Present Practical Modeling Case for You:
1) Mouse Acute Pancreatitis Modeling Method
2) Mouse Acute Pancreatitis Model Evaluate Method
Method: Mouse were fasted but drink freely 12 hours before surgery. Intraperitoneal injection with 25μg/kg dose Ceruletide (a.k.a. INN, caerulein) leads to development of edematous acute pancreatitis (CIP) in 5 hours.
Features: Present edematous acute pancreatitis (CIP), the pancreatitis is comparatively mild
Retrograde infusion of the bile duct involves ligating the pancreaticobiliary duct, inserting a cannula in the main pancreatic duct and injecting a substance (like sodium deoxycholate and autologous bile etc.) such as pancreatin. The injection of pancreatin arouses acute pancreatitis - if perfused with PGE2, it will cause acute necrotizing pancreatitis. Schmidt’s team perfused low-volume glycodeoxycholic acid combined with intravenous caerulein through pancreatic duct to create rat acute pancreatitis model which present well-distributed injury of moderate acinar necrosis, which is suitable for pancreatitis research.
Acute pancreatitis may be induced by ligating the distal bile duct in duodenum or pancreatic duct. Lerch’s team ligated in the ampullary region and edematous pancreatitis appeared in 6 hours, while at 12 hours post-ligation the pancreas presented bleeding, necrosis, inflammatory cell infiltration. The advantage of this modeling is includes avoidance of the drug-induced nonspecific systemic effects and it is similar to human bile reflux pancreatitis. Runzi’s team improved the modeling method - cutting off the duodenum’s proximal end and ligate both ends with common bile duct, lettijg pancreatic juice to flow into the closed loop - as its internal pressure increases, reflux occurs, resulting in acute necrotizing pancreatitis. Edema pancreatitis occurred 4h after operation, and hemorrhage and necrosis occurred 9-12h. In addition, there is also the use of pancreatic duct ligation and pancreatic artery or vein blockade to produce acute pancreatitis.