
“Among several commercial services who have claimed to be specialized to generate mouse models with genetic modifications, I recognize that Cyagen is one of the best. We are looking forward to having more cooperation with them.”
Korea Brain Research Institute (KBRI)We will respond to you in 1-2 business days.
The cell line-derived xenograft (CDX) model, one of the commonly used models for studying tumor cell proliferation and in vivo drug screening, involves inoculation of tumor cells (subcultured in vitro) into immunodeficient mice. Due to the long-term cell passage in vitro, they have the characteristics of high homology, easy construction, and reproducibility. However, in the process of continuous culture and passage in vitro, tumor heterogeneity is quite different from the original tumor tissue.
Cyagen can provide various pharmacological evaluation methods, such as tumor growth curve/weight curve, blood biochemical indicators, pathological test (frozen section and paraffin section examination, H&E staining, immunohistochemistry (IHC), etc.), flow cytometry, molecular testing, which can meet your multiple needs for the analysis of drug mechanism.
Backgrounds: BALB/c nude, NOD scid, C-NKG mice, etc.
Typical Injection Sites: Subcutaneous, intravenous, or in situ.
Cyagen can provide you with various CDX models, such as breast cancer, liver cancer, bowel cancer, pancreatic cancer, lung cancer, stomach cancer, kidney cancer, prostate cancer, melanoma, bladder cancer, leukemia, ovarian cancer, brain cancer models, as well as highly customized in vivo pharmacodynamic services for the corresponding models.
Selected Cell line derived xenografts (CDX) Model Cases
A549 Human non-small cell lung cancer (NSCLC) cell subcutaneous tumor model
The growth curve of A549 Human non-small cell lung cancer cell subcutaneous tumor
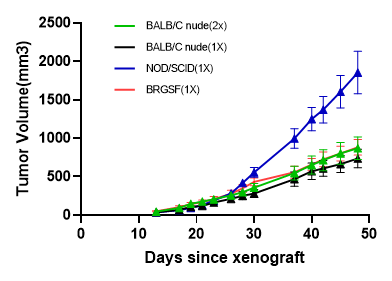
Figure 1. Formation experiment of A549 Human non-small cell lung cancer (NSCLC) cell subcutaneous tumor.
A549 cells were inoculated into BALB/c nude mice, NOD/SCID mice through subcutaneous injection, to measure the tumor volume at different time points.


Figure 2. Photos of nude mice inoculated with A459 cells 6 weeks after inoculation.